Author Affiliations
Abstract
Vonoprazan, a potent inhibitor of gastric acid secretion, is a recently introduced drug. It is debated to be better than or equal to proton pump inhibitors (PPIs) in the treatment of acid-based disorders and Endoscopic Submucosal Dissesction (ESD) induced ulcers. However, its efficacy regarding bleeding control, healing, and gastrointestinal tract (GIT) perforation after ulceration is not been sufficiently studied. The purpose of this study is to determine the efficacy of vonoprazan vs lansoprazole and to compare the side effect profile of both drugs. Data was sourced from PubMed, Google Scholar, and Cochrane databases. The initial search revealed 51 studies. After exclusion, 9 were included. All included studies were randomized controlled trials (RCTs) covering post-ESD treatment with Vonoprazan vs Lansoprazole over a period of 7 years (2016 to 2022), with sample sizes ranging from 14 to 250 subjects. The mean age of subjects was 65.02 years. The diagnostic modality was endoscopy. Study quality was calculated using the Modified Jadad or Oxford Scale. The pooled odds ratio of vonoprazan vs lansoprazole for ulcer healing rate was 1.34 (95% confidence interval [CI],1.00-1.79, I2=50%) with a statistically significant p-value of 0.05. The pooled Odds ratio of vonoprazan vs lansoprazole for bleeding control was 0.55 (95% CI, 0.24-1.27, I2=12%) with a p-value of 0.16, and that of vonoprazan vs lansoprazole for perforation was 1.49 (95% CI, 0.44-5.02, I2=0%), with a p-value of 0.52. Our study concluded that lansoprazole has a better ulcer healing rate than vonoprazan, while both have comparable bleeding and perforation control.
Keywords
Vonoprazan, Lansoprazole, Gastrointestinal tract (GIT), Ulcer, Randomized controlled trials (RCTs), Endoscopy, Bleeding.
Introduction
Gastric and Duodenal ulcers have become increasingly common in recent years, owing either to increased acid secretion or decreased defense mechanisms of the mucosa [1]. The introduction of acid suppression since the 1970s has transformed the treatment of these acid-based gastrointestinal disorders [2]. PPIs have been the treatment of choice for many years. However, they come with their drawbacks and sometimes are refractory in treatment, so a newer class of acid suppressors called Potassium-competitive acid blockers (P-CABs) was developed, which was expected to have a better side effect profile [3].
ESD is a procedure developed in the early 2000s for the resection of mucosal pathologies and, in recent years, it has been widely implemented in Asian countries [4]. However, certain complications may follow in the post-operative period following ESD, which include Hemorrhage, Perforation, and Stenosis [5]. A hemorrhage is a serious post-operative complication and is defined as bleeding that develops within 4 weeks of ESD [6].
Multiple guidelines have recommended the use of PPIs for controlling post-ESD bleeding [7,8,9]. PPIs also improve ESD-induced ulcer healing rate by decreasing gastric acid secretion [10]. A study shows that PPIs should be given for a minimum of 2 weeks post-ESD to promote effective ulcer healing without increasing any adverse effects [11]. However, the CYP2C19 polymorphisms of PPIs were found to alter their pharmacokinetic and pharmacodynamic properties, resulting in variable outcomes of therapy [12]. An alternate drug is needed with more stable pharmacokinetic and pharmacodynamic properties.
Vonoprazan is a potassium-competitive acid blocker that was introduced in February 2015 [13]. It rapidly reduces gastric acid levels and maintains a steady state for 24 hours, thus offering a long-lasting acid suppression [14]. Vonoprazan has proved to be an effective drug for the control of delayed bleeding in post-ESD ulcers by reducing the chances of delayed bleeding in ulcers >2cm, those with a scar, and those located in the antrum [15].
Vonoprazan and Lansoprazole have been compared in studies to determine the drug with better outcomes. A study showed that, inspite of vonoprazan having a faster healing rate in the initial post-ESD period, which was 4 weeks, and PPIs having better healing at 8 weeks, the overall ESD-induced ulcer shrinkage rate and control of bleeding were similar for both drugs [16]. Another study comparing Vonoprazan with Lansoprazole showed no significant difference in ulcer shrinkage rate between the two drugs [17]. While in other settings, Vonoprazon has been found to have better bleeding control post-ESD [18]. There is a need to further evaluate the two drugs in different populations and clinical settings. So, we conducted a meta-analysis that included randomized and non-randomized control trials on patients having any kind of ulcers in the GIT. These patients were aged between 16 and 70 years. Vanoprazan/Lansoprazole was administered to these patients, and a follow-up of a minimum of 7 days was done to determine the control of these drugs on disease progression and their side effects.
Methodology
This systematic review and meta-analysis were done using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [19].
Eligibility criteria
Studies were eligible with consideration of the following inclusion criteria: (1) published full text and in the English language, (2) patients with a GIT ulcer were included, and (3) ulcer reduction rate and bleeding control after endoscopic submucosal dissection was reported with the inclusion of adult (age ≥20 years). (4) The study was included only if it was an RCT. Moreover, studies were carefully assessed if they had provided a comparison between Lansoprazole and Vonoprazan, separately, and were excluded if not. Articles were excluded if they were reviews, editorials, or case reports. The following Table 1 shows the patient population, intervention, comparator, outcomes, timing, and setting (PICOTS) criteria used to assess the eligibility of the studies.
| Population | Age ≥ 20 years |
| Intervention/Exposure | Any kind of ulcer in GIT |
| Comparator | Vonoprazan / Lansoprazole |
| Outcome | v Ulcer healing rate/Shrinkage ratio v Bleeding control v Perforation |
| Time | 7 days follow-up |
| Study design | RCTs and non-RCTs |
Table 1: PICOTS criteria
Search Strategy & Study Selection
A systematic literature search was conducted using PubMed, Google Scholar and Cochrane databases with the following subject keywords and their MeSH terms: ‘Vonoprazan’, ‘Lansoprazole,’ ‘Gastrointestinal Tract Ulcers,’ ‘ESD induced ulcers,’ ‘Gastric ulcers,’ ‘Duodenal Ulcers,’ ‘PPIs’ and ‘P-CABs’ and these were combined with Boolean Operator such as OR/AND. This study was carried on for almost two months from July 27, 2022, to September 24, 2022.
Studies were initially shortlisted based on title and abstract, after which the full text was assessed for eligibility. References of the selected studies were also reviewed thoroughly to prevent any risk of selection bias.
Data Collection Process
Two authors separately reviewed and extracted data from included records, separately using the online systematic review tool: CADIMA. Disagreements were resolved by involving the third author deciding the extraction process according to our study criteria.
Data Items
Data extraction of the relevant studies included the first author, year of publication, type of study (cohort or RCT), study follow-up time, and the total number of patients who underwent treatment for any kind of ulcer treatment. Baseline characteristics and indications for ulcer treatment were also extracted. Primary outcomes of 7-day ulcer reduction and bleeding control rates were extracted by careful screening of tables and results of the individual studies. We only included secondary outcomes if three or more studies reported them. Details are present in the Table.1
Risk of bias in individual studies
Assessment of risk of bias quality assessment of all the randomized controlled studies was done using the oxford quality scoring or Jadad scale. It was done both at the study level and the outcome level. The outcome level bias was assessed using the funnel plot made by the Review Manager. It determines the authenticity of the synthesis. The principal measures were the odds ratio and the difference in means.
Synthesis of Results
The systematic review was done using the CADIMA tool for systematic reviews. All the extracted data were statistically analyzed using the Review Manager. Ulcer healing rate and all the secondary outcomes (delayed bleeding time and perforation) were pooled using both random and fixed effects models. The I2 statistic was used to analyze the heterogeneity, with a value of >75% labeled as severe heterogeneity of the data. In addition to that, we also conducted a multivariate meta-regression analysis to assess the association between the odds ratio and vonprazan and lansoprazole in post-ESD ulcer patients.
Risk of bias in studies
A funnel plot was constructed using Review Manager for assessment of the risk of bias in studies. There was a small publication bias found in the included studies as represented by the funnel plot shown below. The funnel plot shown (Figure 1) is asymmetrical as the studies are not evenly distributed on both sides of the plot.
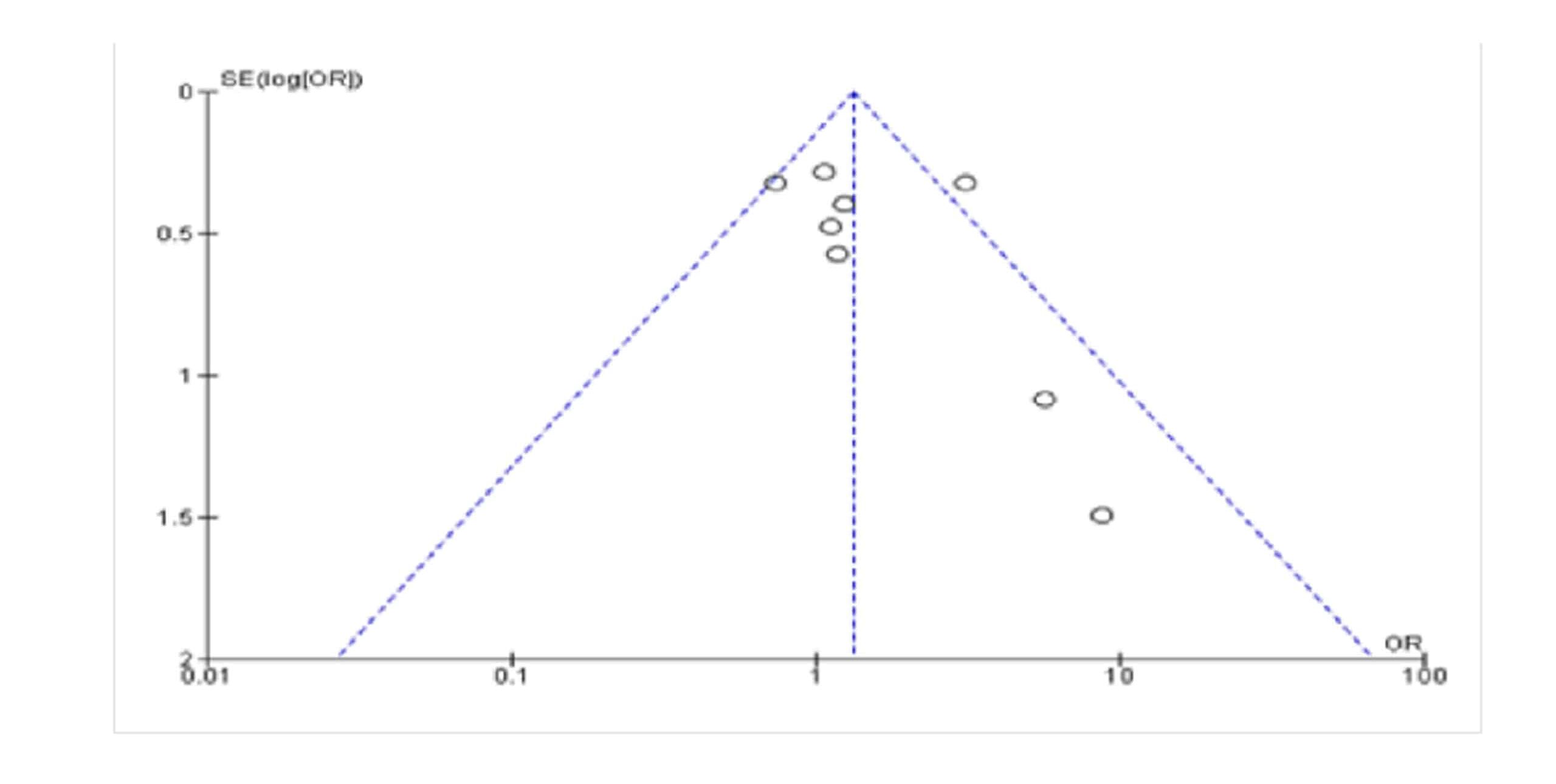
Figure 1: Funnel plot for assessment of publication bias
Results
Study Selection
The initial search revealed 51 studies. After exclusions, 9 studies, published between 2016 and 2022, were included in a systematic review and 8 studies included meta-analysis. The following PRISMA flow diagram (Figure 2) shows the studies excluded at each step of study selection.
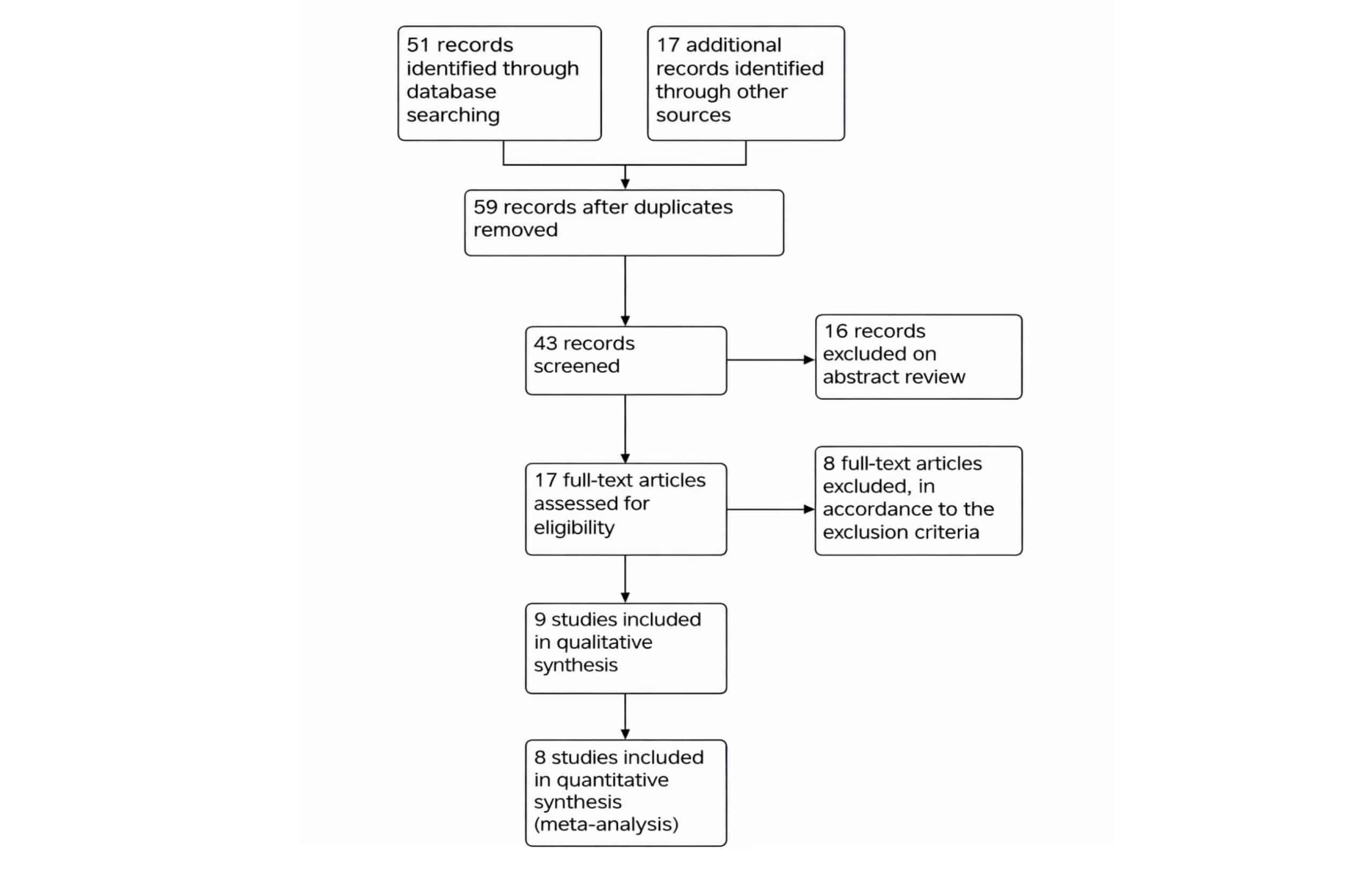
Figure 2: PRISMA flow diagram
Primary Outcome
Ulcer healing rate/shrinkage ratio: The pooled odds ratio of Vonoprazan vs Lansoprazole for ulcer healing rate was 1.34 (95% CI,1.00-1.79, I2=50%), which was statistically significant with a p-value of 0.05 as shown in Figure 3.
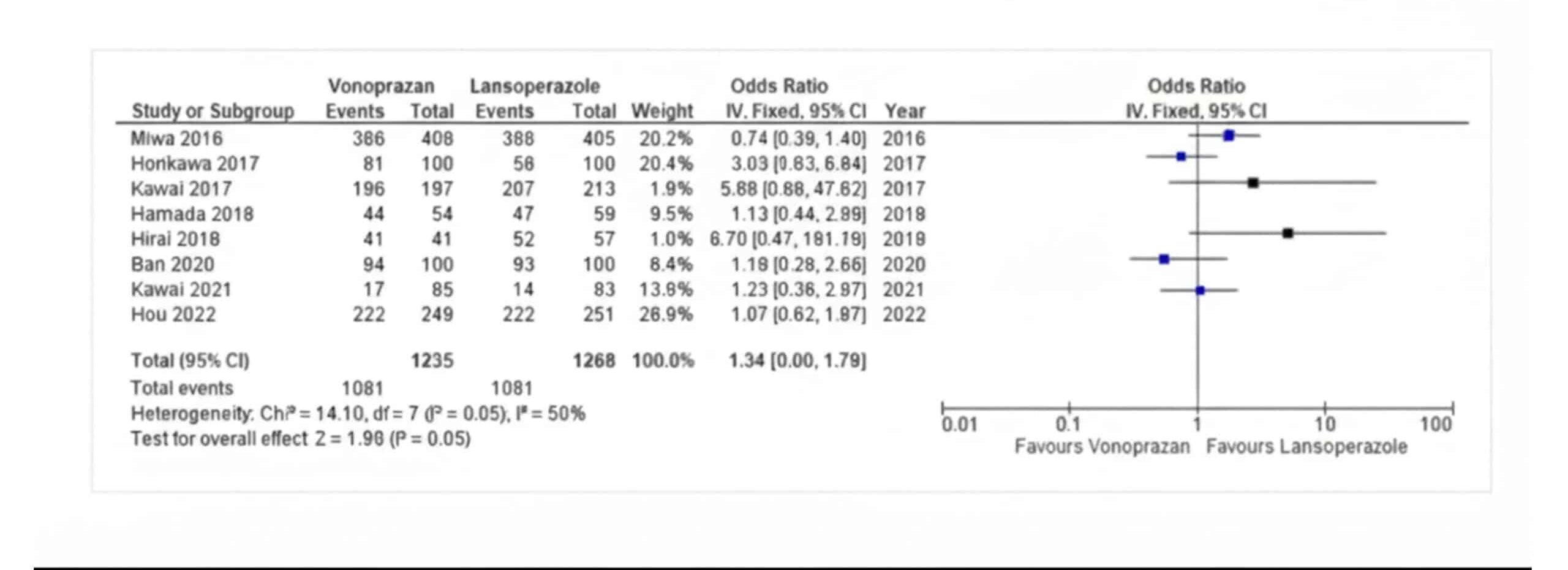
Figure 3: Ulcer healing rate/shrinkage ratio
Secondary Outcome
Bleeding control: The pooled odds ratio of Vonoprazan vs Lansoprazole for bleeding control was 0.55 (95% CI, 0.24-1.27, I2=12%), which was insignificant with a p-value of 0.16 as indicated in Figure 4, studies reporting the bleeding control.
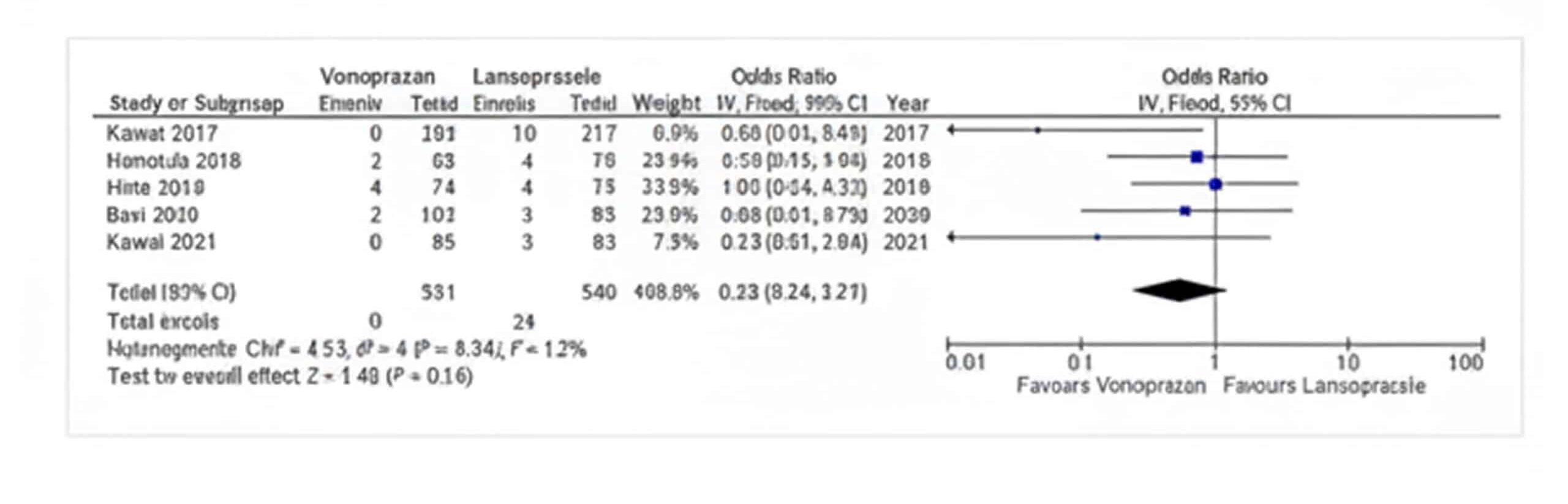
Figure 4: Bleeding control
Perforation: The pooled odds ratio of Vonoprazan vs Lansoprazole for Perforation was 1.49 (95% CI, 0.44-5.02, I2=0%,) which was insignificant with a p-value of 0.52 as indicated in Figure 5, studies reporting the perforation.
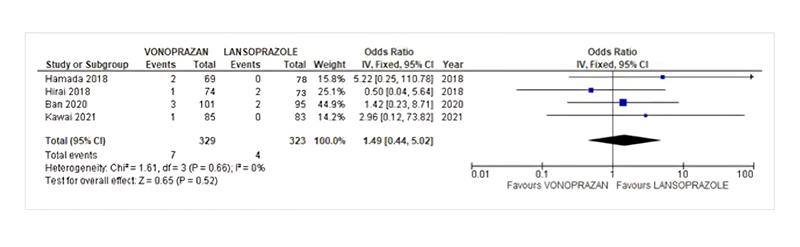
Figure 5: Perforation
| Study (Year) | Mean Age | Sample Size | Kind Of Ulcers in GIT | Dose Of Vonoprazan | Comparator | Followup | Effectiveness
|
Side Effects
Vonoprazan |
Side Effects
Lansoperazole |
Quality Assessment |
| Miwa et al. (2016)
[36] |
>20 years
(54.2 + 14.7) |
814 | Gastric Ulcers / Duodenal Ulcers | 20mg | Lansoprazole 30mg | 8 weeks (GU)
6 weeks (DU) |
-Non-inferiority of vonoprazan to lansoperazole in healed GU and DU (Resolution of heartburn was higher in vonoprazan group) | -Dizziness
– Subarachnoid hemorrhage -DU hemorrhage
|
-Headache
-Acute pancreatitis -Vomiting |
6 |
| Kawai et al. (2017) [37] | > 20 years (68.6 + 8.54)
|
410 | Previous peptic ulcers | 10mg /20mg | Lansoprazole
30mg |
12 and 24 weeks.
Labs every 4 weeks.
|
The proportion of recurrent ulcers and bleeding in GIT at 24 weeks was higher in the lansoperazole group vs vonoprazan groups. The difference was statistically non-significant. It determines the non-inferiority of vonoprazan to lansoperazole. | 10mg group
-Acute pancreatitis -Large intestinal polyp -Cholelithiasis -Thrombotic cerebral infarction -Diabetic neuropathy 20mg group -Gastric cancer -Enterocolitis -Oral fibroma
|
-Acute pancreatitis
-Cervical adenocarcinoma -Acute renal failure |
5 |
| Hamada et al. (2018) [15] | > 20 years (70.2 + 7.35) | 139 | ESD induced gastric ulcers | 20mg | Lansoprazole 30mg | 8 weeks after ESD with endoscopy | Vonoprazan siginificantly lowered the delayed bleeding rate. No significant difference from the lansoperazole group | -Nausea, Vomiting, Diarrhoea
–Perforation -Pneumonia -Gastric stenosis |
-Nausea, Vomiting, Diarrhoea
-Gastric stenosis -Rash |
5 |
| Takashi et al. (2016) [17] | Not reported (73.35 + 8.1) | 26 | ESD induced Ulcers | 20mg | Lansoprazole 30mg | Follow-up on the 8th day and 28th day of ESD. | ESD results:
1) Resection rate, procedure time, histopathology of lesions, frequency of submucosal fibrosis was not statistically different in both groups Evaluation of ESD-induced ulcers and delayed bleeding 2)No statistically different results in both groups. |
Not reported | Not reported | 4 |
| Horikawa et al. (2017) [38] | Not reported (71.25, Median value) | 115 | ESD induced ulcers | 20mg | Lansoprazole 30mg | 12 days | Ulcer reduction at 2 weeks:
1)Vonoprazan showed significantly more reduction in ulcer size as compared to lansoprazole group coverage ratio of ulcer base by granulation tissue at 2 weeks: 2)Vonoprazan showed significantly accelerated results as compared to lansoprazole |
Not reported | Not reported | 5 |
| Hirai et al.
(2018) [29] |
>20 years (71.5 + 9.24) | 149 | ESD induced ulcers | 20mg | Lansoprazole
30mg |
8 weeks | Healing ratio at 4 and 8 weeks:
1)Did not differ significantly between the vonoprazan and lansoprazole group Shrinkage ratio: 2)Not statistically different between the two groups
|
-Delayed Bleeding of no significant importance
-Perforation in 1 patient |
-Delayed bleeding of no significant importance
-Perforation in 2 patients |
4 |
| Kawai et al. (2021) [22] | >20 years (73, Median value) | 168 | ESD induced Ulcers | 20mg | Lansoprazole 30mg | 8 weeks | Shrinkage ratio:
1)No statistically significant difference between vonoprazan and lansoprazole group
|
-Delayed perforation | -Post-operative bleeding | 5 |
| Ban et al. (2020)
[39] |
>20 years (71.35 + 8.7) | 196 | ESD induced ulcers | 20mg | Lansoprazole 30mg | 8 weeks | 1)No significant differences in the reduction rate of ulcers in both groups.
2)Complete healing ratio did not differ significantly between both groups
|
-Delayed bleeding | -Delayed Bleeding | 5 |
| Hou et al. (2022)
[40] |
>18 years (41.7 + 12.54) | 533 | Duodenal ulcers | 20mg twice daily | Lansoprazole 30mg | 10 weeks | 1)Non inferiority of vonoprazan versus lansoprazole for endoscopic healing of duodenal ulcers was confirmed
2)Vonoprazan was non-inferior to lansoprazole for H.pylori eradication at 4 weeks. 3)Resolution rates were comparable between both groups |
-Diarrhoea
-Increased Blood Gastrin -Increased Pepsinogen
|
-Increased pepsinogen
-Upper respiratory Tract infection
|
7 |
Table 2: Data extraction sheet
Discussion
The debate about Vonoprazan, a potent inhibitor of gastric acid secretion, being better than PPIs, one of the most commonly prescribed groups of medications, in the treatment of acid-based disorders or ESD is far from over [20]. A lot of clinical trials have established the fact that vonoprazan is non-inferior, if not superior, to all other kinds of medications used in gastric acid-induced disorders while having a better side effect profile as well [21]. Despite all the trials done on the drug, its efficacy regarding ulcer healing, bleeding control, and gastrointestinal perforation for ulcers due to ESD remains unclear [22]. Therefore, a systematic review and meta-analysis were conducted to understand the cumulative result of these individual trials.
ESD is a widely used tool in Asia and has been gaining popularity in other parts of the world as well. Which is why most of our studies have been done in Japan and China [23]. ESD has significantly reduced morbidity and mortality in patients, in comparison to the surgical procedures used in the past [24]. The efficacy of ESD is determined by postoperative ulcer healing, bleeding control, and several other factors [25]. To prevent these postoperative side effects and increase the efficacy of this procedure, potent acid inhibition is required [26]. PPIs have always been the mainstay of treatment for acid inhibition, all over the world [27]. Potassium-competitive acid blocker(P-CAB), a newly discovered drug group for acid inhibition, is expected to do better than even PPIs [28].
Our study concluded that Lansoprazole (PPI) has a significantly superior ulcer healing rate in ESD-induced gastric ulcers, as compared to Vonoprazan (P-CAB). In contrast to the studies done before, which concluded that the Shrinkage ratio/Ulcer Healing Ratio is similar in Vonoprazan and Lansoprazole [22,29,30,31]. Our study determined that Lansoprazole is superior as far as the shrinkage ratio is concerned. Our study also negates the previously presented evidence that Vonoprazan has a better ulcer healing ratio as compared to lansoprazole [32]. This might be due to the fact that there is no clinically significant effect of CYP2C19 polymorphisms on vanoprazazan. Within 4 hours of administration, vonoprazan starts exerting its acid inhibitory effect by raising intragastric pH to 4, and the acid suppression sustains for 24 hours [33]. Conversely, PPIs have clinically significant effects of CYP2C19 polymorphism (CYP2C19 IMs and PMs having better efficacy than NMs) and a half-life of up to 5 hours, due to which they need to be administered two to three times a day [34].
Our study showed that Vonoprazan and Lansoprazole had equal efficacy in controlling post-ESD bleeding, as supported by the work done previously as well [29,35]. It also disapproves of the work of previous studies, stating the superiority of lansoprazole [22]. Similarly, our study demonstrated that Vonoprazan and Lansoprazole have no significant difference in controlling perforation, which was in accordance with the previous data [22]. However, only 5 studies were used to assess these two drugs for bleeding control, and only 4 studies could be assessed for perforation. There is a need to include a larger number of studies on more diverse populations around the world, so the comparison between the two classes of drugs can be generalized better.
The limitations of the study are 1) Only 9 RCTs could be included in our study after eligibility assessment, and most of them were conducted in Japan. Including a larger and more generalized population may show results different from our study. 2) The polymorphisms of CYP2C19 could not be assessed in our study, which may have altered the results. 3)The difference in post-ESD ulcer sizes may yield variable results. The sizes were not assessed in our study. 4) Subjects who were on medications like anticoagulants, antiplatelets, and steroids should have been excluded from our study, as the complications resulting from their use could have varied the results.
Conclusion
Our study showed that Lansoprazole had better ulcer healing and shrinkage ratio as compared to Vonoprazan. Moreover, both Vonoprazan and Lansoprazole had similar efficacy in controlling post-ESD bleeding and perforation. This study contributes to a better understanding of the efficacy and side effect profile of the newer P-CAB, Vanoprazan, as compared to the traditional PPIs. It further encourages healthcare providers to administer and study this drug’s efficacy in various other populations, including more subjects.
References
- Dajani EZ, Klamut MJ. Novel therapeutic approaches to gastric and duodenal ulcers: an update. Expert Opin Investig Drugs. 2000;9(7):1537-1544. doi:10.1517/13543784.9.7.1537 PubMed | Crossref | Google Scholar
- Bodemar G, Walan A. Cimetidine in the treatment of active duodenal and prepyloric ulcers. Lancet. 1976;2:161-164. doi:10.1016/S0140-6736(76)92342-4 PubMed | Crossref | Google Scholar
- Scarpignato C, Hongo M, Wu JCY, Lottrup C, Lazarescu A, Stein E, Hunt RH. Pharmacologic treatment of GERD: Where we are now, and where are we going? Ann NY Acad Sci. 2020;1482(1):193-212. doi:10.1111/nyas.14473 PubMed | Crossref | Google Scholar
- Nishimura M. ESD and Pit Pattern Diagnosis: Lessons from a Japanese Endoscopist Working in the United States. Clin Colon Rectal Surg. 2020;33(6):329-334. doi:10.1055/s-0040-1714235 PubMed | Crossref | Google Scholar
- Bai Y, Cai JT, Chen YX, et al. Expert consensus on perioperative medications during endoscopic submucosal dissection for gastric lesions (2015, Suzhou, China). J Dig Dis. 2016;17(12):784-789. doi:10.1111/1751-2980.12430 PubMed | Crossref | Google Scholar
- Yang CH, Qiu Y, Li X, Shi RH. Bleeding after endoscopic submucosal dissection of gastric lesions. J Dig Dis. 2020;21(3):139-146. doi:10.1111/1751-2980.12850 PubMed | Crossref | Google Scholar
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47(9):829-854. doi:10.1055/s-0034-1392882 PubMed | Crossref | Google Scholar
- Fernandez-Esparrach G, Calderon A, De-la-Pena J, et al. Endoscopic submucosal dissection. Rev Esp Enferm Dig. 2014;106(2):120-132. doi:10.4321/S1130-01082014000200007 PubMed | Crossref | Google Scholar
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28(1):3-15. doi:10.1111/den.12518 PubMed | Crossref | Google Scholar
- Ko J, Kim SJ, Kang DH, Choi CW, Kim HW, Park SB. Dose-related healing of artificial ulcers after endoscopic submucosal dissection using esomeprazole: A randomized controlled study. Medicine (Baltimore). 2019;98(20):e15701. doi:10.1097/MD.0000000000015701 PubMed | Crossref | Google Scholar
- Niimi K, Fujishiro M, Goto O, et al. Prospective single-arm trial of two-week rabeprazole treatment for ulcer healing after gastric endoscopic submucosal dissection. Dig Endosc. 2012;24(2):110-116. doi:10.1111/j.1443-1661.2011.01178.x PubMed | Crossref | Google Scholar
- Furuta T, Shirai N, Sugimoto M, et al. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet. 2005;20(3):153-167. doi:10.2133/dmpk.20.153 PubMed | Crossref | Google Scholar
- Hori Y, Imanishi A, Matsukawa J. 1-(5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335:231-238. doi:10.1124/jpet.110.170274 PubMed | Crossref | Google Scholar
- Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41(7):636-648. doi:10.1111/apt.13121 PubMed | Crossref | Google Scholar
- Hamada K, Uedo N, Tonai Y, et al. Efficacy of vonoprazan in prevention of bleeding from endoscopic submucosal dissection-induced gastric ulcers: a prospective randomized phase II study. J Gastroenterol. 2019;54(2):122-130. doi:10.1007/s00535-018-1487-6 PubMed | Crossref | Google Scholar
- Kang H, Kim BJ, Choi G, Kim JG. Vonoprazan versus proton pump inhibitors for the management of gastric endoscopic submucosal dissection-induced artificial ulcer: A systematic review with meta-analysis. Medicine (Baltimore). 2019;98(24):e15860. doi:10.1097/MD.0000000000015860 PubMed | Crossref | Google Scholar
- Takahashi K, Sato Y, Kohisa J, et al. Vonoprazan 20 mg vs lansoprazole 30 mg for endoscopic submucosal dissection-induced gastric ulcers. World J Gastrointest Endosc. 2016;8(19):716-722. doi:10.4253/wjge.v8.i19.716 PubMed | Crossref | Google Scholar
- Shiratori Y, Niikura R, Ishii N, et al. Vonoprazan versus proton pump inhibitors for post-endoscopic submucosal dissection bleeding in the stomach: a multicenter population-based comparative study. Gastrointest Endosc. 2022;95(1):72-79.e3. doi:10.1016/j.gie.2021.06.032 PubMed | Crossref | Google Scholar
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097 PubMed | Crossref | Google Scholar
- Ban H, Inatomi O, Murata M, et al. Vonoprazan vs lansoprazole for the treatment of artificial gastric ulcer after endoscopic submucosal dissection: a prospective randomized comparative study. J Clin Biochem Nutr. 2021;68(3):259-263. doi:10.3164/jcbn.20-143 PubMed | Crossref | Google Scholar
- Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: A dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015. doi:10.1111/apt.13331 PubMed | Crossref | Google Scholar
- Kawai D, Takenaka R, Ishiguro M, et al. Vonoprazan versus lansoprazole in the treatment of artificial gastric ulcers after endoscopic submucosal dissection: a randomized, open-label trial. BMC Gastroenterol. 2021;21(1):236. doi:10.1186/s12876-021-01822-5 PubMed | Crossref | Google Scholar
- Harlow C, Sivananthan A, Ayaru L, et al. Endoscopic submucosal dissection: an update on tools and accessories. Ther Adv Gastrointest Endosc. 2020;13. doi:10.1177/2631774520957220 PubMed | Crossref | Google Scholar
- Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic submucosal dissection: indications and application in Western endoscopy practice. Gastroenterology. 2018;154(7):1887-1900.e5. doi:10.1053/j.gastro.2018.01.068 PubMed | Crossref | Google Scholar
- Uraoka T, Ochiai Y, Fujimoto A, et al. A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest Endosc. 2016;83(6):1259-1264. doi:10.1016/j.gie.2015.11.015 PubMed | Crossref | Google Scholar
- Huh CW, Youn YH, Jung DH, et al. Early attempts to eradicate Helicobacter pylori after endoscopic resection of gastric neoplasm significantly improve eradication success rates. PLoS One. 2016;11(9):e0162258. doi:10.1371/journal.pone.0162258 PubMed | Crossref | Google Scholar
- Kajiura S, Hosokawa A, Ueda A, et al. Effective healing of endoscopic submucosal dissection-induced ulcers by a single week of proton pump inhibitor treatment: a retrospective study. BMC Res Notes. 2015;8. doi:10.1186/s13104-015-1111-2 PubMed | Crossref | Google Scholar
- Kagawa T, Iwamuro M, Ishikawa S, et al. Vonoprazan prevents bleeding from endoscopic submucosal dissection-induced gastric ulcers. Aliment Pharmacol Ther. 2016;44(6):583-591. doi:10.1111/apt.13747 PubMed | Crossref | Google Scholar
- Hirai A, Takeuchi T, Takahashi Y, et al. Comparison of the effects of vonoprazan and lansoprazole for treating endoscopic submucosal dissection-induced artificial ulcers. Dig Dis Sci. 2018;63(4):974-981. doi:10.1007/s10620-018-4948-0 PubMed | Crossref | Google Scholar
- Ban H, Inatomi O, Murata M, et al. Vonoprazan vs lansoprazole for the treatment of artificial gastric ulcer after endoscopic submucosal dissection: a prospective randomized comparative study. J Clin Biochem Nutr. 2021;68(3):259-263. doi:10.3164/jcbn.20-143 PubMed | Crossref | Google Scholar
- Harada S, Takeuchi T, Ozaki H, et al. Mo1148 Efficacy of Vonoprazan Compared With Lansoprazole On the Healing Of Artificial Gastric Ulcers After Endoscopic Submucosal Dissection: A Prospective, Randomized Controlled Trial. Gastrointest Endosc. 2018;63(4):974-981. doi:10.1016/j.gie.2018.04.1909 Crossref
- Horikawa Y, Mizutamari H, Mimori N, et al. Short-term efficacy of potassium-competitive acid blocker following gastric endoscopic submucosal dissection: a propensity score analysis. Scand J Gastroenterol. 2018;53(2):243-251. doi:10.1080/00365521.2017.1410569 PubMed | Crossref | Google Scholar
- Echizen H. The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations. Clin Pharmacol Ther. 2016;55(4):409-418. doi:10.1007/s40262-015-0326-7 PubMed | Crossref | Google Scholar
- Lima JJ, Thomas CD, Barbarino J, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin Pharmacol Ther. 2021;109(6):1417-1423. doi:10.1002/cpt.2015 PubMed | Crossref | Google Scholar
- Harada S, Takeuchi T, Ozaki H, et al. Mo1148 Efficacy of Vonoprazan Compared With Lansoprazole On The Healing Of Artificial Gastric Ulcers After Endoscopic Submucosal Dissection: A Prospective, Randomized Controlled Trial. Gastrointest Endosc. 2018;63(4):974-981. doi:10.1016/j.gie.2018.04.1909 Crossref
- Miwa H, Uedo N, Watari J, et al. Randomised clinical trial: efficacy and safety of vonoprazan vs. lansoprazole in patients with gastric or duodenal ulcers – results from two phase 3, non-inferiority randomised controlled trials. Aliment Pharmacol Ther. 2017;45(2):240-252. doi:10.1111/apt.13876 PubMed | Crossref | Google Scholar
- Kawai T, Oda K, Funao N, et al. Vonoprazan prevents low-dose aspirin-associated ulcer recurrence: randomised phase 3 study. Gut. 2018;67(6):1033-1041. doi:10.1136/gutjnl-2017-314852 PubMed | Crossref | Google Scholar
- Horikawa Y, Mizutamari H, Mimori N, et al. Short-term efficacy of potassium-competitive acid blocker following gastric endoscopic submucosal dissection: a propensity score analysis. Scand J Gastroenterol. 2018;53(2):243-251. doi:10.1080/00365521.2017.1410569 PubMed | Crossref | Google Scholar
- Ban H, Inatomi O, Murata M, et al. Vonoprazan vs lansoprazole for the treatment of artificial gastric ulcer after endoscopic submucosal dissection: a prospective randomized comparative study. J Clin Biochem Nutr. 2021;68(3):259-263. doi:10.3164/jcbn.20-143 PubMed | Crossref | Google Scholar
- Hou X, Meng F, Wang J, et al. Vonoprazan non-inferior to lansoprazole in treating duodenal ulcer and eradicating Helicobacter pylori in Asian patients. J Gastroenterol Hepatol. 2022;37(7):1275-1283. doi:10.1111/jgh.15837 PubMed | Crossref | Google Scholar
Acknowledgments
The authors, Ameer Hamza Mahmood, Nehala Nooz, and Syed Muhammad Jawad Zaidi, extend their gratitude to all individuals who contributed to the development of this manuscript.
Funding
The authors declare that no financial funding was received.
Author Information
Corresponding Author:
Ameer Hamza Mahmood-ul-Hassan
Department of Medicine
Rawalpindi Medical University, Lahore, Punjab, Pakistan
Email: zamziorpion@gmail.com
Co-Authors:
Nehala Nooz, Syed Muhammad Jawad Zaidi
Department of Medicine
Rawalpindi Medical University, Lahore, Punjab, Pakistan
Authors Contributions
Syed Muhammad Jawad Zaidi: Investigation, All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation and writing – review & editing to refine the manuscript. Additionally, all authors participated in the Supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Informed Consent
Not applicable
Conflict of Interest Statement
None
Guarantor
Not reported
DOI
Cite this Article
Ameer Hamza M-ul-H, Nehala N, Jawad Zaidi SM. Vonoprazan vs Lansoprazole in Gastrointestinal Tract Ulcers: A Systematic Review and Meta-Analysis. medtigo J Med. 2024;2(3):e3062239. doi:10.63096/medtigo3062239 Crossref









