Author Affiliations
Abstract
Ribose-5-phosphate isomerase (RPI) is a key enzyme in the pentose phosphate pathway (PPP), which plays a critical role in cellular metabolism by balancing nucleotide synthesis and redox homeostasis. RPI catalyzes the reversible conversion between ribose-5-phosphate (R5P) and ribulose-5-phosphate (Ru5P), an essential reaction for rapidly proliferating cells. RPI exists in two isoforms, RPIA and RPIB, which exhibit distinct structural and functional characteristics across different organisms. Recent studies have highlighted the physiological relevance of RPI in maintaining cellular redox balance and its role in neurodegenerative diseases and cancer metabolism. Moreover, RPI has emerged as a potential therapeutic target, with inhibitors showing promise in disrupting the metabolic processes crucial for tumor cell proliferation and pathogenic microorganism survival. This review explores RPI’s structure, function, and regulatory mechanisms, its pathological implications, and ongoing research into its therapeutic potential. Future research directions are also discussed, focusing on the enzyme’s role in oxidative stress, neurodegeneration, and antimicrobial resistance.
Keywords
Ribose-5-phosphate isomerase, Pentose phosphate pathway, Redox homeostasis, Antimicrobial resistance.
Introduction
RPI: A key enzyme in cellular metabolism
RPI is an essential enzyme in the PPP, a critical metabolic route responsible for nucleotide synthesis and maintaining cellular redox balance.[1] The enzyme catalyzes the reversible conversion between R5P and Ru5P, two pentose phosphates integral to cellular metabolism. This reaction is particularly vital in rapidly dividing cells, such as those in proliferative tissues, where the demand for nucleotides is high.[2] RPI is expressed in two primary isoforms, RPIA and RPIB, which have distinct structural and kinetic properties. RPIA is more commonly found in plants and certain bacteria, whereas RPIB exists in other organisms, including various bacterial and fungal species.[3] Both isoforms share a conserved catalytic mechanism that involves the formation of a high-energy enediolate intermediate during the isomerization process.[4]
Structural features of RPI isomerase
RPI typically functions as a homodimer, with each subunit containing an active site that facilitates the reversible interconversion of R5P and Ru5P.[5] The catalytic site is comprised of key amino acid residues, including conserved histidine and aspartate residues, which are critical for substrate binding and catalysis. Mutations in these residues can severely impair enzyme function, leading to metabolic disorders and altered substrate specificity.[6] Crystallographic studies have revealed that the enzyme adopts a TIM-barrel fold, a common structural motif found in many enzymes involved in sugar metabolism. This structure allows for flexibility in substrate binding, which is essential for the enzyme’s function under varying cellular conditions.[7]
Role of RPI in the pentose phosphate pathway
The pentose phosphate pathway is divided into two phases: the oxidative phase, which produces nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), and the non-oxidative phase, where RPI plays a key role. RPI ensures the balance between nucleotide synthesis and the recycling of sugar phosphates back into glycolysis. By facilitating the conversion of R5P to Ru5P, RPI regulates the flow of carbon through the PPP, which is critical for cells undergoing rapid growth, such as cancer cells.[8] Moreover, the production of NADPH in the oxidative branch of the PPP is essential for anabolic processes and for maintaining the cellular redox state.[9]
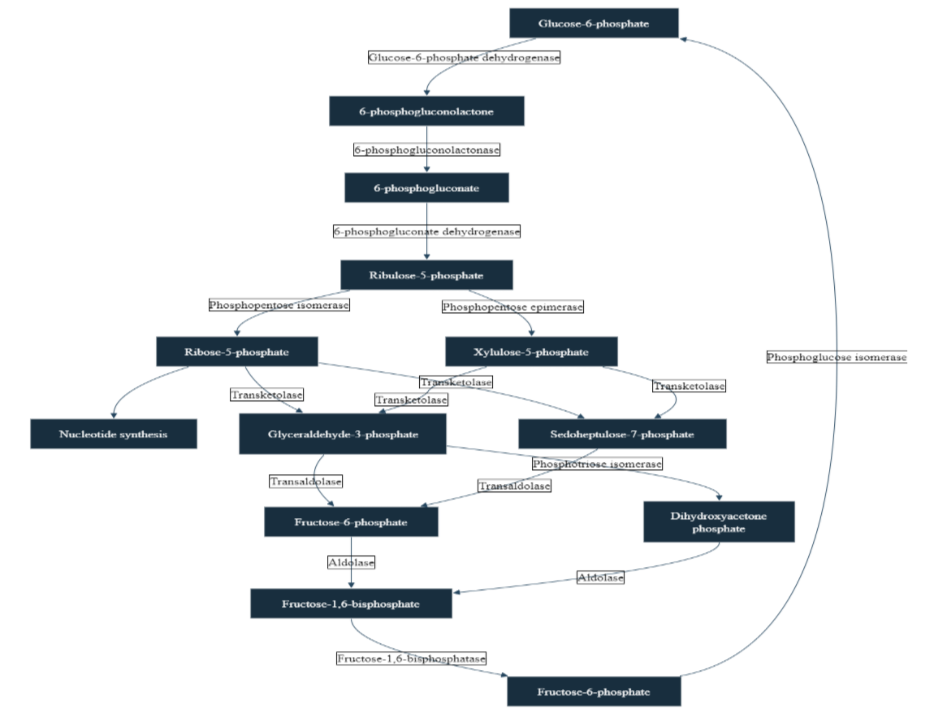
Figure 1: The diagram illustrates the complex Pentose Phosphate Pathway, a metabolic process crucial for cellular function
It’s organized into two distinct phases: the oxidative phase, depicted in the upper portion of the image, and the non-oxidative phase, shown in the lower section. Throughout the diagram, key metabolites are represented as nodes, while the enzymes catalyzing each reaction are indicated on the arrows connecting these nodes. The pathway produces several important compounds: Ribose-5-phosphate, which is essential for nucleotide synthesis; NADPH, which, although not visually represented, is a vital product of the oxidative phase; and Fructose-6-phosphate and Glyceraldehyde-3-phosphate, both of which can feed into the glycolysis pathway. A notable feature of the PPP is its cyclical nature, illustrated by an arrow that loops from Fructose-6-phosphate back to the starting compound, Glucose-6-phosphate, demonstrating the pathway’s ability to regenerate its initial substrate.
Physiological and pathological relevance
RPI’s function is not limited to metabolism; the enzyme is implicated in regulating cell signaling pathways and responding to oxidative stress.[5] In particular, RPI has been shown to play a role in neurodegenerative diseases and metabolic disorders characterized by elevated levels of polyols, such as ribitol and arabitol, in body fluids.[11] The accumulation of these metabolites results from defects in the PPP and can lead to neurological impairments, including developmental delays and motor dysfunction.[12]
RPI and cancer metabolism
Alterations in PPP enzyme activities, including RPI, are frequently observed in cancer cells. The upregulation of the PPP supports the increased demand for nucleotide synthesis and NADPH production in rapidly proliferating tumor cells.[13] Targeting RPI or other PPP enzymes may offer therapeutic opportunities in oncology, as disrupting this pathway can limit the biosynthetic capabilities of cancer cells and enhance their sensitivity to oxidative stress.[14]
Discussion
Future directions in RPI research
Ongoing research into RPI’s structure and function continues to unveil new therapeutic possibilities. Targeting RPI with small-molecule inhibitors could help manage conditions related to metabolic imbalances, such as cancer and neurological disorders. Advances in structural biology have opened the door to designing specific inhibitors that block the enzyme’s activity without affecting other metabolic pathways.[15]
RPI in oxidative stress and redox homeostasis
RPI plays a pivotal role in maintaining cellular redox balance, largely through its involvement in the non-oxidative branch of the pentose phosphate pathway. This pathway generates NADPH, a critical reducing agent that helps protect cells against oxidative stress by regenerating reduced glutathione, one of the body’s most important antioxidants.[8] The importance of NADPH extends to anabolic processes, including fatty acid synthesis and the detoxification of reactive oxygen species (ROS).[17] Consequently, defects in RPI can compromise the cell’s ability to maintain redox homeostasis, resulting in increased susceptibility to oxidative damage. In cancer cells, which often experience elevated levels of oxidative stress, the pentose phosphate pathway is upregulated, with enzymes like RPI playing a crucial role in managing the increased demand for both nucleotides and reducing power. This adaptation allows cancer cells to survive in hostile environments characterized by elevated ROS levels.[18] Targeting RPI or other PPP enzymes to reduce NADPH production has been suggested as a potential therapeutic strategy to weaken cancer cells’ defenses against oxidative stress.[14]
Therapeutic potential of targeting RPI
Given its critical role in multiple metabolic and regulatory pathways, RPI has emerged as a potential therapeutic target in both metabolic disorders and cancer. Inhibiting RPI activity could disrupt the balance of the pentose phosphate pathway, limiting nucleotide production and impairing cell proliferation, especially in tumor cells that rely on heightened metabolic activity for growth and survival.[9] Furthermore, inhibiting RPI may reduce the capacity of cancer cells to mitigate oxidative stress, making them more vulnerable to chemotherapy and radiation therapy, which work in part by inducing oxidative damage.[21]
In addition to oncology, RPI inhibitors may have therapeutic potential in metabolic diseases where dysregulation of the pentose phosphate pathway leads to pathological accumulation of metabolites. Such diseases include conditions associated with aberrant polyol metabolism, where selective RPI inhibition might prevent the accumulation of toxic intermediates.[12] The development of selective RPI inhibitors, however, remains a challenge due to the enzyme’s highly conserved active site and its structural similarity to other sugar isomerases. Advances in structure-based drug design, along with high-resolution crystallography, may help overcome this obstacle, allowing for the development of small molecules that selectively target RPI without affecting other metabolic enzymes.[4]
As the understanding of RPI’s role in metabolism and disease expands, several important areas of future research emerge. One such area is the elucidation of RPI’s regulatory mechanisms, both at the transcriptional and post-translational levels. While significant progress has been made in understanding RPI’s catalytic activity, the mechanisms by which its activity is regulated in response to cellular and environmental changes remain poorly understood.[13] This knowledge could provide new avenues for therapeutic intervention, particularly in diseases where RPI activity is dysregulated. Another promising area of research involves exploring RPI’s interactions with other enzymes in the pentose phosphate pathway. Studies have shown that metabolic enzymes often function as part of larger multi-enzyme complexes, or metabolons, which allow for more efficient substrate channeling and regulation.[2] Investigating whether RPI forms such complexes could provide insights into how its activity is coordinated with other PPP enzymes, potentially uncovering new therapeutic targets.
Finally, research into the genetic regulation of RPI may help identify individuals who are at risk for metabolic disorders or cancers associated with pentose phosphate pathway dysfunction. Understanding how mutations in the RPI gene affect enzyme activity and metabolic flux could lead to the development of personalized therapeutic strategies based on individual metabolic profiles.[26]
Genetic variations and disease associations
Mutations in the RPI gene can lead to severe metabolic disorders, including a rare condition known as RPI deficiency. This disorder is characterized by accumulating polyols such as ribitol and arabitol in tissues and bodily fluids, which can disrupt normal metabolic functions.[11] Patients with this deficiency often exhibit symptoms such as developmental delays, neurological deficits, and progressive leukoencephalopathy. These clinical manifestations are thought to arise due to impaired nucleotide synthesis and oxidative stress, resulting from the dysfunctional pentose phosphate pathway.[19]
The first case of RPI deficiency was identified in a patient with progressive neurological deterioration, highlighting the critical role of the enzyme in maintaining brain metabolism.[3] Since then, only a few additional cases have been reported, indicating the rarity of the disorder. However, these cases underscore the importance of understanding the genetic basis of RPI activity, as even small variations in enzyme function can have profound metabolic consequences. Research into the genetic regulation of RPI is still in its early stages. Still, studies suggest that single-nucleotide polymorphisms (SNPs) in the RPI gene may contribute to variations in enzyme activity and metabolic efficiency. These findings could have significant implications for personalized medicine, as individuals with reduced RPI activity may be at higher risk for metabolic disorders or conditions related to oxidative stress.[20]
RPI’s role in neurological health
The neurological symptoms associated with RPI deficiency provide important insights into the enzyme’s broader physiological roles, particularly in the brain. The brain is highly dependent on glucose metabolism for energy production and nucleotide synthesis, and disruptions in the pentose phosphate pathway can have devastating effects on neuronal function.[12] RPI deficiency results in an imbalance of metabolic intermediates, leading to the accumulation of toxic byproducts and an inability to maintain adequate levels of NADPH, which is essential for managing oxidative stress in neurons.[14]
Animal models of RPI deficiency have demonstrated that elevated levels of polyols can interfere with mitochondrial function, leading to increased oxidative stress and neuronal damage. These findings suggest that therapies aimed at restoring RPI activity or reducing polyol accumulation could help mitigate the neurological effects of the disorder.[8] Given the growing evidence of RPI’s involvement in neurodegenerative conditions, further research into its role in brain metabolism could open new therapeutic avenues for treating disorders such as alzheimer’s disease, parkinson’s disease, and other neurodegenerative diseases where oxidative stress plays a key role.[5]
Exploring RPI as a therapeutic target
The discovery of RPI’s role in both normal physiology and disease has opened up new opportunities for therapeutic intervention. The dual role of RPI in nucleotide synthesis and oxidative stress management suggests that targeted manipulation of this enzyme could offer therapeutic benefits in various conditions, including cancer and metabolic disorders. Recent advances in enzyme inhibition and drug design have allowed researchers to begin exploring RPI inhibitors as potential treatments.
One area of interest is the development of small-molecule inhibitors that selectively target RPI in cancer cells. Cancer cells are heavily reliant on the pentose phosphate pathway for nucleotide synthesis and redox balance, making RPI an attractive target for drug development. By inhibiting RPI, it may be possible to disrupt the metabolic processes that support cancer cell growth, ultimately leading to cell death or enhanced sensitivity to traditional chemotherapeutic agents.[9]
In addition to cancer, targeting RPI could provide therapeutic relief for metabolic diseases. Conditions like RPI deficiency are characterized by the toxic buildup of polyols and oxidative stress. By developing treatments that restore or modulate RPI activity, it may be possible to alleviate the metabolic imbalances that contribute to the disease phenotype.[19]
Challenges in targeting RPI
Despite the promise of targeting RPI for therapeutic purposes, several challenges remain. One significant challenge is the enzyme’s structural similarity to other sugar isomerases, which makes selective inhibition difficult. Achieving specificity in drug design is crucial to avoid unintended effects on other metabolic pathways. Furthermore, RPI’s role in essential metabolic processes raises concerns about the potential side effects of enzyme inhibition, particularly in non-cancerous tissues where nucleotide synthesis and redox balance are also critical.[3]
To overcome these challenges, researchers are exploring advanced drug design techniques, including structure-based drug design and high-throughput screening. The use of X-ray crystallography and molecular modeling has provided detailed insights into the active site of RPI, allowing for the rational design of inhibitors that can selectively bind to this enzyme without affecting other metabolic enzymes.[4] Another avenue of exploration is the use of allosteric inhibitors, which can modulate enzyme activity by binding to sites other than the active site. This approach could provide a way to fine-tune RPI activity without completely blocking its function, potentially minimizing the risk of side effects.[7]
Advances in RPI research and diagnostic applications
In recent years, advancements in genomics and metabolic profiling have enhanced our understanding of RPI and its broader role in human health. New diagnostic techniques, such as next-generation sequencing (NGS) and metabolomic analyses, have made it possible to detect RPI-related metabolic disorders earlier, enabling more targeted interventions.[16] These advancements are particularly important for rare diseases such as RPI deficiency, where early diagnosis can significantly improve patient outcomes. For instance, high-throughput metabolomic screening can identify abnormal levels of polyols like ribitol and D-arabitol in biological samples, which are indicative of RPI deficiency.[2] Such biomarkers not only aid in diagnosing the condition but can also serve as a tool to monitor disease progression and the effectiveness of potential treatments. Genetic testing through NGS can further confirm the presence of mutations in the RPI gene, helping to identify at-risk individuals or carriers within affected families.[19]
Moreover, gene therapy holds promise as a future treatment for genetic disorders linked to RPI deficiencies. The successful restoration of RPI activity in affected individuals could prevent the onset of metabolic imbalances that lead to neurological impairments. While gene therapy is still in the experimental stage, advances in clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 (Cas9) technology and viral vector systems have raised hopes for treating genetic conditions by directly correcting faulty genes.[8]
RPI in the context of systems biology
The study of RPI has also benefited from advances in systems biology, which integrates genomics, proteomics, and metabolomics to gain a comprehensive understanding of complex biological networks. By mapping the interactions between RPI and other enzymes in the pentose phosphate pathway, researchers have better understood how RPI influences overall cellular metabolism.[13] Systems biology approaches have revealed that the pentose phosphate pathway, where RPI plays a key role, is intricately connected to other metabolic pathways, such as glycolysis and fatty acid synthesis. The interplay between these pathways highlights the importance of RPI in maintaining cellular homeostasis, particularly in tissues with high metabolic demands, such as the liver, brain, and rapidly proliferating tumor cells.[14] This interconnectedness suggests that perturbations in RPI activity could have far-reaching effects on multiple metabolic processes, further underscoring the importance of tightly regulating RPI activity within the cell.
Emerging therapeutic strategies
As RPI continues to gain attention as a therapeutic target, researchers are exploring innovative strategies to modulate its activity. One emerging approach is the use of RNA-based therapies, such as antisense oligonucleotides (ASOs) and RNA interference (RNAi), which can downregulate the expression of RPI in cancer cells.[3] By specifically targeting the mRNA encoding RPI, these therapies could reduce the enzyme’s activity in tumor cells without affecting its function in normal tissues, thereby minimizing off-target effects.
In parallel, enzyme replacement therapies (ERTs) are being investigated as a potential treatment for metabolic disorders caused by RPI deficiencies. ERTs have been successful in treating other enzyme deficiencies, such as Gaucher disease, by providing patients with a recombinant form of the missing or deficient enzyme. While still in the experimental phase for RPI-related conditions, ERT holds promise as a way to restore normal metabolic function in affected individuals.[20]
Additionally, immunotherapy approaches are being explored to target RPI in cancer. By identifying specific epitopes on RPI that are overexpressed in tumor cells, researchers aim to develop vaccines or monoclonal antibodies that can selectively attack cancer cells without harming healthy tissues. These immune-based therapies could provide a novel way to target metabolic vulnerabilities in cancer cells, offering a complementary strategy to traditional chemotherapy and radiation.[9]
RPI and metabolic flexibility in pathogenic microorganisms
In addition to its role in human metabolism and disease, RPI has been implicated in the metabolic flexibility of pathogenic microorganisms. Pathogens such as Toxoplasma gondii and Trypanosomatids rely on the PPP to adapt to different environmental conditions and maintain the necessary balance of nucleotides and redox power for survival in host organisms.[14] The non-oxidative branch of the PPP, where RPI plays a central role, is essential for pathogen survival, particularly in fluctuating environments where the availability of glucose and other nutrients is limited. For example, studies on Trypanosomatids, which are responsible for diseases such as leishmaniasis and Chagas disease, have revealed that RPI is critical for the pathogen’s ability to generate R5P for nucleotide biosynthesis and to maintain oxidative stress homeostasis.[3] Inhibiting RPI in these organisms could disrupt their metabolic processes, rendering them vulnerable to oxidative damage and reducing their ability to proliferate within their host.
Moreover, in microbial pathogens, RPI is often subject to regulatory mechanisms that allow the pathogen to prioritize energy and biomass production during times of nutrient scarcity. This metabolic adaptability is key to the pathogen’s survival in the host environment, making RPI a promising target for antimicrobial therapies. Recent studies suggest that selective RPI inhibitors could serve as potent antimicrobial agents by exploiting the pathogen’s reliance on the PPP.[20]
Potential for Targeting RPI in Antimicrobial Therapy
The development of antimicrobial agents that target RPI has garnered significant interest, particularly in light of the growing problem of antimicrobial resistance. Targeting enzymes like RPI, which are essential for nucleotide synthesis and redox balance in microbial pathogens, offers a promising approach for novel antimicrobial therapies. By disrupting the metabolic pathways that these pathogens rely on, RPI inhibitors could limit pathogen survival without directly targeting traditional cellular components, such as bacterial cell walls or ribosomes, which have been the focus of many current antibiotics.[9]
Furthermore, because RPI is highly conserved across a range of pathogenic organisms, inhibitors developed to target RPI in one pathogen may be effective against a broader spectrum of microbial species. For instance, targeting RPI in Trypanosomatids could also have applications in treating infections caused by other protozoan parasites or even bacterial pathogens that rely on the pentose phosphate pathway for survival under oxidative stress.[8]
Challenges and considerations in antimicrobial RPI inhibition
Despite the potential for targeting RPI in antimicrobial therapy, several challenges must be addressed. One major challenge is achieving selective inhibition of RPI in pathogens without affecting the enzyme’s function in human cells. While RPI is conserved across many species, subtle differences in the enzyme’s active site between pathogens and humans can be exploited to develop selective inhibitors. Structural studies comparing the RPI of pathogens with that of humans could help identify such differences and guide the design of selective drugs.[5]
Another consideration is the risk of developing resistance to RPI inhibitors. As with any antimicrobial therapy, the potential for pathogens to evolve resistance must be carefully managed. Combining RPI inhibitors with other antimicrobial agents could reduce the likelihood of resistance by simultaneously targeting multiple metabolic pathways. Moreover, using RPI inhibitors in combination with existing antibiotics could enhance the overall efficacy of treatment by weakening the pathogen’s ability to survive under oxidative stress and nutrient-limiting conditions.[4]
Future directions in RPI-based antimicrobial research
Moving forward, research into RPI-based antimicrobial therapies will need to focus on the identification of small-molecule inhibitors that can selectively target pathogen-specific RPI without disrupting human metabolism. High-throughput screening of chemical libraries, coupled with structure-based drug design, holds promise for discovering novel compounds that can inhibit RPI in microbial pathogens. Additionally, further elucidation of the regulatory mechanisms governing RPI activity in pathogens may uncover new ways to enhance the specificity and efficacy of RPI-targeted therapies.[7]
Given the role of RPI in maintaining nucleotide balance and redox homeostasis in a variety of pathogens, including protozoa, fungi, and bacteria, targeting this enzyme represents an exciting avenue for the development of next-generation antimicrobials. These treatments could be particularly valuable in combating drug-resistant infections, where traditional therapies have proven ineffective.
RPI and its role in host-pathogen interactions
The interplay between host and pathogen metabolism is an increasingly important area of research, and RPI is no exception. Pathogens rely on the host’s metabolic resources to thrive, and RPI, being a critical enzyme in the pentose phosphate pathway, is integral to this process. Studies have shown that during infection, pathogens often hijack host metabolic pathways, including the PPP, to sustain their growth and survival.[14]
For instance, intracellular parasites such as Toxoplasma gondii are known to manipulate host cell metabolism to increase the availability of metabolic intermediates, including those generated via the pentose phosphate pathway. By upregulating RPI activity in host cells, the parasites ensure a steady supply of RPI for their nucleotide biosynthesis, facilitating rapid proliferation.[8] Understanding how pathogens exploit host RPI offers potential strategies for therapeutic intervention, where disrupting the pathogen’s access to host metabolic pathways could limit its ability to survive and replicate.
Immune evasion and RPI
Recent studies suggest that RPI may also play a role in immune evasion strategies employed by certain pathogens. For instance, some bacterial pathogens can modulate the host’s immune response by altering its redox balance, which is closely tied to the pentose phosphate pathway.[21] By influencing the activity of RPI and other PPP enzymes, pathogens can increase the production of NADPH, a key molecule in the host’s antioxidant defenses. This modulation of the host’s redox environment helps the pathogen to avoid detection and destruction by immune cells. Pathogens that can manipulate host RPI may be able to delay or subvert immune responses, allowing for more prolonged and successful infections. Targeting these metabolic interactions could provide a novel approach to enhancing the host immune response and clearing infections more effectively.[9]
Implications for vaccine development
Given RPI’s central role in pathogen survival and host-pathogen interactions, there is growing interest in exploring this enzyme as a target for vaccine development. In the context of certain bacterial and protozoan infections, RPI and other PPP enzymes are essential for the pathogen’s metabolic processes. Vaccines that target key components of pathogen metabolism, such as RPI, could elicit an immune response that inhibits the pathogen’s ability to generate nucleotides and maintain redox homeostasis, thereby reducing its capacity to survive in the host.[3] For example, studies on Mycobacterium tuberculosis have identified RPI as a potential vaccine candidate due to its essential role in the bacterium’s pentose phosphate pathway. Immunization strategies that focus on metabolic enzymes like RPI could offer a new way to prevent or treat infections by impairing the pathogen’s ability to thrive within the host.[4]
Role of RPI in drug-resistant pathogens
With the rise of drug-resistant pathogens, targeting metabolic pathways like the PPP has emerged as an innovative strategy to overcome resistance. Traditional antibiotics often target the bacterial cell wall, protein synthesis machinery, or DNA replication. However, as pathogens evolve mechanisms to resist these drugs, there is a growing need for alternative targets. RPI, being a key player in the pentose phosphate pathway, offers a promising target for combating drug-resistant infections.[5] By inhibiting RPI, researchers aim to disrupt the energy and biosynthetic needs of resistant bacteria, effectively starving them of the metabolic resources required for growth and replication. This approach could be particularly effective against pathogens like Staphylococcus aureus and Escherichia coli, which have developed resistance to multiple classes of antibiotics.[27] RPI inhibitors could be used in combination with existing antibiotics to enhance their effectiveness and overcome resistance mechanisms by exploiting the pathogen’s metabolic vulnerabilities.
RPI in non-infectious diseases: neurodegeneration and beyond
While much of the focus on RPI research has been on its role in cancer and infectious diseases, recent studies suggest that RPI may also be implicated in non-infectious conditions such as neurodegenerative diseases. Neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases, are often associated with mitochondrial dysfunction and oxidative stress, both of which are tightly linked to the pentose phosphate pathway.[16] RPI’s role in maintaining cellular redox balance through the production of NADPH may be critical in neurons, which are particularly vulnerable to oxidative damage. Disruptions in the pentose phosphate pathway can exacerbate oxidative stress in the brain, leading to neuronal damage and disease progression. Investigating RPI’s role in neurodegeneration could provide new insights into the metabolic basis of these disorders and identify potential therapeutic strategies aimed at restoring metabolic balance in affected neurons.[12]
RPI as a biomarker for disease progression
In addition to its potential as a therapeutic target, RPI is emerging as a potential biomarker for several diseases. Biomarkers are invaluable in the early detection, diagnosis, and monitoring of disease progression, particularly in cancer and metabolic disorders. Alterations in RPI activity and expression levels have been observed in several cancers, suggesting that RPI could serve as a biomarker for tumor aggressiveness or therapeutic response.[14] For example, in rapidly proliferating tumor cells, there is often an upregulation of the pentose phosphate pathway, including enzymes like RPI, to meet the increased demand for nucleotides and maintain redox homeostasis.[16] Elevated levels of RPI have been associated with poor prognosis in certain cancers, such as breast and colorectal cancer, where enhanced PPP activity correlates with increased tumor growth and metastasis.[9] Monitoring RPI levels could therefore provide clinicians with insights into tumor behavior and help guide treatment decisions, particularly in selecting therapies that target metabolic vulnerabilities.
In metabolic disorders, particularly those involving disruptions in the pentose phosphate pathway, RPI activity could also serve as a biomarker for disease severity. For instance, patients with RPI deficiency, a rare genetic disorder, exhibit abnormal levels of pentose metabolites in their tissues and bodily fluids, which could be used to track disease progression and response to enzyme replacement therapies.[2] By establishing standardized assays for measuring RPI activity in clinical samples, it may be possible to develop diagnostic tests that aid in the early detection and management of metabolic disorders.
RPI in aging and cellular senescence
Another emerging area of research involves the role of RPI in aging and cellular senescence. Cellular senescence is a state of permanent cell cycle arrest that occurs in response to various stressors, including DNA damage, oxidative stress, and telomere shortening. Senescent cells are characterized by metabolic reprogramming, including alterations in the pentose phosphate pathway, which helps these cells manage oxidative stress and maintain nucleotide pools.[5] RPI plays a crucial role in this process by supporting the non-oxidative branch of the PPP, which is involved in generating R5P for nucleotide synthesis. Studies suggest that RPI activity may decline with age, contributing to the reduced proliferative capacity of senescent cells and the accumulation of oxidative damage in aging tissues.[8] Furthermore, the accumulation of ROS in aging cells can overwhelm the antioxidant defenses provided by the pentose phosphate pathway, exacerbating cellular damage and promoting the onset of age-related diseases, such as neurodegeneration and cancer.
Modulating RPI activity to restore metabolic balance in aging cells represents a potential therapeutic strategy for delaying the onset of age-related diseases. By boosting the activity of RPI and other PPP enzymes, it may be possible to enhance the production of NADPH and other metabolites that protect cells from oxidative stress, thus promoting healthy aging and reducing the risk of chronic diseases.[11]
RPI in mitochondrial function and energy metabolism
RPI has also been implicated in mitochondrial function and energy metabolism. The pentose phosphate pathway, although cytosolic, interacts closely with mitochondrial metabolic processes, particularly through the generation of NADPH, which is essential for mitochondrial antioxidant defenses and the maintenance of mitochondrial DNA integrity.[3] Mitochondria are the primary source of cellular energy production, and their function is tightly regulated by metabolic pathways, including the PPP. Disruptions in RPI activity can lead to imbalances in cellular energy metabolism, contributing to mitochondrial dysfunction. This dysfunction is particularly evident in neurodegenerative diseases, where mitochondrial deficits play a central role in disease pathology. For example, in Alzheimer’s disease and Parkinson’s disease, mitochondrial oxidative stress and energy deficits are thought to drive neuronal damage and cognitive decline.[19] By modulating RPI activity to enhance mitochondrial antioxidant capacity and energy production, it may be possible to mitigate some of the metabolic deficits associated with these diseases.
Moreover, the relationship between RPI and mitochondrial function extends to metabolic diseases such as diabetes, where disruptions in energy metabolism and increased oxidative stress contribute to disease progression. Targeting RPI and the pentose phosphate pathway may offer new therapeutic strategies for restoring mitochondrial function and improving metabolic health in these conditions.[12]
Novel therapeutic approaches: RPI modulation and gene therapy
As the therapeutic potential of RPI becomes more apparent, novel approaches to modulating its activity are being explored. One promising avenue is gene therapy, particularly for genetic disorders like RPI deficiency. Gene therapy offers the potential to restore normal RPI function in affected individuals by delivering a functional copy of the RPI gene to the patient’s cells. Advances in CRISPR-Cas9 and viral vector technologies have made gene therapy more feasible, and preclinical studies are already demonstrating the efficacy of this approach in animal models of metabolic disorders.[4] Another area of interest is the development of small molecules that can modulate RPI activity. Unlike gene therapy, which directly alters the genetic makeup of a patient, small-molecule drugs offer a less invasive approach to regulating enzyme activity. High-throughput screening techniques are being employed to identify compounds that can either enhance or inhibit RPI activity, depending on the therapeutic goal. For instance, in cancer, where RPI activity is often upregulated, inhibitors that selectively block RPI could be used to impair tumor metabolism and limit cancer growth.[2]
Conversely, in metabolic and neurodegenerative diseases, where RPI activity may be compromised, small molecules that enhance RPI function could help restore metabolic balance and improve cellular health. These drugs could be used alone or in combination with other therapies, such as antioxidants or mitochondrial-targeted treatments, to achieve synergistic effects.[9]
Expanding RPI research beyond human health: Agricultural and industrial applications
In addition to its relevance in human health, RPI has garnered interest in agricultural and industrial biotechnology. The ability of RPI to influence sugar metabolism makes it a key enzyme in plants and microorganisms used in industrial processes such as biofuel production and biomass conversion. Research into the metabolic engineering of microorganisms to optimize RPI activity could have significant implications for improving the efficiency of biofuel production by maximizing the utilization of carbon sources like glucose and xylose.[21]
In agriculture, enhancing the activity of RPI in plants could lead to improved growth and stress resilience. Since the pentose phosphate pathway contributes to the production of nucleotides and reducing equivalents, increasing RPI activity may boost plant growth rates and enhance their ability to withstand environmental stressors such as drought and high salinity. This could have implications for food security, as crops with optimized metabolic pathways could yield higher outputs under challenging conditions.[22]
RPI in synthetic biology and biotechnology: The expanding field of synthetic biology also offers exciting opportunities for the application of RPI. By integrating RPI into engineered metabolic pathways, researchers can create microorganisms with enhanced capabilities for producing valuable chemicals, pharmaceuticals, and biofuels. The enzyme’s role in generating R5P, an essential precursor for nucleotide biosynthesis, makes it an attractive target for synthetic biology applications aimed at producing nucleotides and nucleoside-based drugs.[23]
Moreover, advancements in enzyme engineering have enabled the modification of RPI to improve its catalytic efficiency and stability, making it more suitable for industrial applications. By creating variants of RPI that are more tolerant to extreme conditions such as high temperature or acidity, researchers can develop enzymes that function optimally in industrial bioprocesses, potentially lowering production costs and improving overall efficiency.[24]
RPI as a target for environmental and bioremediation applications: Beyond its roles in agriculture and industry, RPI is also gaining attention in environmental science, particularly in the field of bioremediation. Bioremediation involves the use of microorganisms to break down or remove environmental pollutants, and metabolic pathways such as the PPP are central to this process. By leveraging the metabolic flexibility of microorganisms, including their use of RPI in the PPP, researchers are exploring ways to enhance the degradation of toxic compounds and improve the sustainability of bioremediation strategies.[25]
Microorganisms engineered to have elevated RPI activity may be better equipped to handle the oxidative stress associated with the breakdown of environmental pollutants. Many toxic compounds, such as heavy metals and organic pollutants, generate ROS during their degradation. The PPP, through its generation of NADPH, plays a critical role in managing oxidative stress, and RPI is essential for maintaining the flow of intermediates through this pathway. By enhancing RPI’s role in these microorganisms, it may be possible to boost their resistance to oxidative damage and improve their efficacy in detoxifying polluted environments.[26]
Engineering microbial consortia for environmental cleanup: One promising approach in bioremediation involves the use of microbial consortia, communities of microorganisms that work together to break down complex pollutants. By engineering microbial consortia with optimized RPI and other PPP enzymes, researchers can create more robust systems for environmental cleanup. In such consortia, different species of microorganisms could specialize in various steps of pollutant degradation, with RPI playing a pivotal role in sustaining their energy and redox balance throughout the process.[10] For example, in the degradation of polycyclic aromatic hydrocarbons (PAHs), which are commonly found in contaminated soil and water, microbial consortia with enhanced PPP activity, including upregulated RPI, could more efficiently break down these compounds into less toxic metabolites. This approach offers a sustainable solution to pollution management by using natural metabolic processes to reduce environmental toxins.[27,28]
RPI in the circular economy-recycling and waste reduction: The circular economy concept emphasizes the need for sustainable production processes that minimize waste and recycle resources. RPI, as a key enzyme in microbial metabolism, could play a role in the development of recycling technologies, particularly in the conversion of organic waste into valuable bioproducts. Microorganisms engineered with optimized PPP enzymes, including RPI, could be used in the conversion of agricultural and industrial waste into biofuels, bioplastics, and other biochemicals, thus contributing to waste reduction and resource recovery.[6]
For instance, lignocellulosic biomass, a common byproduct of agricultural activities, can be converted into biofuels through microbial fermentation. RPI helps facilitate this process by contributing to the generation of pentose sugars, which are essential intermediates in the conversion of biomass into ethanol and other fuels. By improving the efficiency of RPI in these metabolic pathways, it may be possible to increase biofuel yields and reduce the environmental footprint of energy production.[21]
Future directions for RPI in environmental biotechnology: As environmental challenges such as pollution and resource scarcity continue to grow, the role of enzymes like RPI in sustainable technologies will become increasingly important. Future research in environmental biotechnology will likely focus on the optimization of RPI and other metabolic enzymes in microorganisms to improve their ability to degrade pollutants, recycle waste, and produce biofuels. Advanced techniques such as CRISPR-based gene editing, directed evolution, and synthetic biology could be employed to engineer microorganisms with enhanced metabolic capabilities. By fine-tuning the expression and activity of RPI, researchers can create microbial systems that are tailored to specific environmental applications, such as bioremediation of oil spills, waste recycling, or carbon capture.[23]
RPI and climate change mitigation-Carbon capture and sequestration: As global efforts to combat climate change intensify, researchers are exploring innovative biological approaches to carbon capture and sequestration. RPI may play a role in microbial systems designed to capture and convert atmospheric carbon dioxide (CO₂) into organic compounds. Microorganisms that utilize the PPP are well-suited for carbon fixation because this pathway generates intermediates essential for biosynthesis, including RPI, which contributes to the formation of nucleotides and other biomolecules.[12]
Engineered microorganisms, such as cyanobacteria and other photosynthetic organisms, can be modified to enhance their carbon fixation capabilities by optimizing RPI and other enzymes within the PPP. These organisms can convert CO₂ into biomass or biofuels, which not only helps reduce the concentration of greenhouse gases in the atmosphere but also creates renewable energy sources. By enhancing RPI activity in these engineered organisms, researchers aim to boost their efficiency in capturing and sequestering carbon, offering a biologically based approach to mitigating the effects of climate change.[23]
Harnessing RPI for carbon sequestration in soil: Another potential application of RPI is in soil carbon sequestration, a process that involves capturing atmospheric CO₂ and storing it in soil through biological, chemical, and physical processes. Plants and soil microorganisms play a central role in this process, as they capture CO₂ during photosynthesis and convert it into organic matter. Enhancing the metabolic pathways, including the PPP, that support this process could improve the soil’s capacity to store carbon.[25] Microorganisms with optimized RPI activity could be introduced into soils to accelerate the conversion of CO₂ into stable organic compounds. This process not only helps to reduce atmospheric CO₂ levels but also improves soil health by increasing the organic content, which can enhance nutrient availability and water retention in agricultural lands. In this context, RPI acts as a crucial metabolic enzyme that supports the microbial processes needed for effective carbon sequestration in the environment.[3]
RPI in marine ecosystems and carbon cycling: Marine ecosystems also play a significant role in the global carbon cycle, with oceans acting as major carbon sinks. Marine microorganisms, particularly phytoplankton, contribute to carbon cycling by fixing CO₂ during photosynthesis and converting it into organic carbon, which eventually sinks to the ocean floor and is stored in sediments. RPI and other enzymes involved in the pentose phosphate pathway are essential for the metabolic processes that support this carbon fixation and the production of biomolecules needed for marine life.[26]
Enhancing RPI activity in marine microorganisms could improve their ability to capture and store carbon, thereby increasing the ocean’s capacity as a carbon sink. Research in this area is focused on understanding the metabolic networks of marine phytoplankton and other microorganisms and identifying ways to optimize these processes to mitigate climate change. By genetically engineering marine microorganisms to enhance their PPP activity, including RPI, researchers aim to increase the efficiency of biological carbon capture in marine ecosystems.[21]
Future directions for RPI in climate change solutions: As the urgency to address climate change grows, research into biological solutions for carbon capture and sequestration is likely to expand. RPI, as a key enzyme in carbon metabolism, will play a role in the development of microorganisms and plants engineered to capture CO₂ and convert it into useful products or store it in stable forms. Advances in synthetic biology and metabolic engineering will allow for the precise manipulation of RPI and other enzymes, optimizing their function in various carbon-capture systems. Additionally, integrating RPI into artificial photosynthesis systems, biological or synthetic constructs that mimic the natural process of photosynthesis, could offer a novel approach to reducing CO₂ levels. These systems could be used to capture carbon from industrial emissions or atmospheric sources and convert it into fuels or chemicals, providing both environmental and economic benefits.[24]
RPI and artificial photosynthesis- A pathway to sustainable energy: One of the most exciting applications of RPI is its potential role in artificial photosynthesis systems. Artificial photosynthesis seeks to mimic the natural process by which plants convert sunlight, water, and CO₂ into energy-rich compounds such as glucose. By utilizing engineered microorganisms or synthetic systems, researchers aim to replicate this process in a controlled environment to produce renewable energy or store carbon. The pentose phosphate pathway, in which RPI plays a pivotal role, is crucial for generating the intermediates needed for both natural and artificial photosynthesis. This pathway produces NADPH, which is essential for converting CO₂ into organic compounds. By optimizing RPI within these systems, it may be possible to increase the efficiency of artificial photosynthesis, enabling the production of biofuels or other chemicals on an industrial scale.[15]
Recent advances in synthetic biology have enabled the design of “biohybrid” systems that combine biological enzymes like RPI with non-biological components to enhance the capture and conversion of solar energy. These systems could help overcome some of the limitations of natural photosynthesis, such as low energy conversion efficiency and limited scalability. By incorporating RPI into these biohybrid systems, researchers can create more efficient pathways for carbon fixation and energy production.[18]
Enhancing biofuel production with RPI: In addition to its role in artificial photosynthesis, RPI can be leveraged to improve biofuel production. The generation of biofuels from renewable biomass sources, such as lignocellulose or algae, has the potential to reduce reliance on fossil fuels and lower greenhouse gas emissions. One of the key challenges in biofuel production is optimizing the conversion of biomass into fermentable sugars, which can then be used to produce ethanol or other biofuels. RPI contributes to this process by enabling the efficient metabolism of pentose sugars, which are abundant in lignocellulosic biomass. The enzyme catalyzes the conversion of R5P into intermediates that are fed into glycolysis, supporting the fermentation process. Enhancing RPI activity in biofuel-producing microorganisms, such as yeast or bacteria, could improve the efficiency of biomass conversion and increase biofuel yields.[10]
In algae-based biofuel systems, RPI also plays a role in carbon fixation, as algae rely on photosynthesis to capture CO₂ and convert it into biomass. By engineering algae to express higher levels of RPI or more efficient variants of the enzyme, it may be possible to accelerate the growth of algae and increase their biofuel-producing capacity. These advancements in metabolic engineering could help make biofuels a more viable alternative to fossil fuels, contributing to a more sustainable energy future.[11]
RPI in biomanufacturing: Production of high-value compounds. Another promising application of RPI is in biomanufacturing, where microorganisms are engineered to produce high-value chemicals, such as pharmaceuticals, amino acids, and polymers. By harnessing the pentose phosphate pathway, which supplies the necessary precursors for nucleotide and amino acid synthesis, RPI helps support the metabolic needs of these biomanufacturing systems. For example, the production of nucleotides and nucleoside-based drugs requires a steady supply of R5P, an intermediate generated by RPI. Enhancing RPI activity in production strains can increase the yield of these compounds, improving the efficiency and cost-effectiveness of the manufacturing process.[18]
RPI plays a role in the production of certain amino acids, such as tryptophan, which are used as feed additives or as precursors in pharmaceutical synthesis. By optimizing the metabolic pathways involving RPI, it may be possible to increase the yield of these valuable compounds.[13] Moreover, RPI can be integrated into synthetic biology platforms to produce biopolymers, such as polyhydroxyalkanoates (PHAs), which are biodegradable alternatives to traditional plastics. The NADPH needed for PHA synthesis, and enhancing RPI activity, can improve the production efficiency of these bioplastics, making them more commercially viable.[4]
Industrial scale-up of RPI-driven processes: One of the major challenges in translating the benefits of RPI research from the laboratory to industrial applications is scaling up these processes to meet commercial demand. While metabolic engineering of microorganisms to enhance RPI activity has shown promise at the bench scale, industrial biomanufacturing requires the optimization of fermentation conditions, feedstocks, and process control to achieve high yields at a large scale. Advances in bioreactor design, process modeling, and synthetic biology tools are helping to address these challenges. For instance, by using computer models to simulate metabolic fluxes in engineered microorganisms, researchers can identify bottlenecks in the metabolic pathways involving RPI and implement targeted interventions to improve overall productivity. Additionally, advances in bioprocessing technology, such as continuous fermentation and automated process control, allow for more efficient scaling up of RPI-driven biomanufacturing systems.[5]
RPI and synthetic biology-Creating customized metabolic pathways: Synthetic biology has emerged as a powerful tool for designing and constructing new biological systems that perform specific functions. RPI plays a crucial role in this field due to its central function in the PPP, which provides the precursors for nucleotide biosynthesis and redox balance. By engineering customized metabolic pathways that incorporate RPI, scientists can create microorganisms with enhanced biosynthetic capabilities for producing biofuels, pharmaceuticals, and other valuable compounds.[21]
One promising application of synthetic biology is the development of microbial cell factories, where engineered microbes are used to produce specific products at industrial scales. These cell factories rely on optimized metabolic pathways to efficiently convert feedstocks, such as sugars, into desired products. By integrating RPI into these synthetic pathways, researchers can increase the availability of R5P, a critical intermediate, and enhance the overall production of nucleotides, amino acids, and biopolymers.[23] For example, microorganisms engineered to overexpress RPI have shown improved yields in the production of nucleotide-based drugs and biochemicals. Additionally, by manipulating the flow of metabolites through the PPP, scientists can redirect carbon flux toward specific biosynthetic routes, making the production of target compounds more efficient.[26]
Engineering RPI for improved performance: As synthetic biology advances, there is a growing interest in improving the catalytic efficiency, stability, and specificity of enzymes like RPI. Directed evolution, a technique that mimics natural selection in the laboratory, allows researchers to evolve enzymes with enhanced properties that are better suited for industrial applications. By subjecting RPI to rounds of mutation and selection, scientists can generate variants with improved activity under a range of conditions, such as higher temperatures, extreme pH levels, or in the presence of industrial solvents.[24] In addition to directed evolution, protein engineering techniques such as site-directed mutagenesis allow for the rational design of RPI with specific modifications that improve its performance. For instance, altering key residues in the enzyme’s active site can increase its affinity for substrates or enhance its catalytic turnover rate, making it more efficient in biotechnological processes. These engineered variants of RPI are particularly valuable for large-scale industrial applications, where enzyme performance directly impacts process efficiency and product yield.
Expanding the substrate scope of RPI
Another area of interest in RPI research involves expanding the enzyme’s substrate scope to enable it to catalyze reactions with non-natural substrates. By engineering RPI to accept a wider range of sugar phosphates, researchers can create novel metabolic pathways that produce new types of chemicals or biomolecules. This approach has significant implications for the bioproduction of rare sugars, which are used as precursors in the synthesis of specialty chemicals, pharmaceuticals, and nutraceuticals.[8] For example, rare sugars such as D-allose and L-xylose have applications in medicine and food science, but their production is often limited by the availability of efficient biosynthetic pathways. By engineering RPI to convert alternative pentose phosphates into these rare sugars, scientists can develop new biocatalytic processes that are more sustainable and cost-effective than traditional chemical synthesis.[12]
RPI in metabolic flux analysis
Cancer therapy: Cancer cells exhibit altered metabolic states, often referred to as the “Warburg effect,” where glucose is preferentially utilized via aerobic glycolysis rather than oxidative phosphorylation. This metabolic reprogramming supports the high rate of nucleotide synthesis required for rapid cancer cell proliferation. One of the key metabolic pathways involved in this process is the PPP, and by targeting enzymes like RPI, it may be possible to disrupt the flow of metabolites necessary for nucleotide biosynthesis and tumor growth.[16]
In many cancers, the PPP is upregulated to generate R5P for nucleotide synthesis and NADPH to manage oxidative stress. By inhibiting RPI, researchers aim to cut off the supply of R5P, thereby limiting the cancer cell’s ability to replicate. This approach may also increase the oxidative stress burden on cancer cells, making them more susceptible to treatments such as chemotherapy and radiation, which induce ROS.[14] Several studies have shown that the inhibition of RPI in cancer cells results in impaired proliferation and increased apoptosis, particularly in cancers with high metabolic demands, such as leukemia, breast cancer, and glioblastoma. Researchers are now exploring small-molecule inhibitors that can specifically block RPI activity in tumor cells, offering a potential new class of anticancer drugs.[3]
Metabolic disorders: In addition to cancer, metabolic disorders such as diabetes and obesity are linked to dysregulation of the PPP. NADPH, a critical product of this pathway, plays an important role in lipid synthesis and glucose metabolism. By modulating RPI activity, it may be possible to restore balance to these metabolic processes in patients with metabolic disorders. In diabetes, for instance, insulin resistance leads to oxidative stress, which exacerbates the disease by promoting further glucose dysregulation and damaging cells. Since the PPP is responsible for producing NADPH, enhancing RPI activity could increase the availability of this antioxidant molecule, helping to reduce oxidative damage and improve metabolic function. Preliminary research has shown that boosting PPP activity, including RPI, can enhance glucose metabolism and improve insulin sensitivity in animal models of diabetes.[2]
In obesity, where lipid synthesis and storage are dysregulated, targeting RPI could also have therapeutic benefits. By modulating the balance of glucose metabolism and lipid synthesis via the PPP, it may be possible to reduce excess fat accumulation and improve overall metabolic health. Further studies are needed to explore the full potential of RPI modulation in the treatment of metabolic diseases.[2]
Antimicrobial drug development: The importance of RPI in the metabolic pathways of various pathogens has also spurred interest in targeting this enzyme for the development of new antimicrobial drugs. Many bacteria and protozoan parasites rely on the PPP for nucleotide biosynthesis and oxidative stress management, making RPI a promising target for drug development. For example, in Mycobacterium tuberculosis (the causative agent of tuberculosis), the PPP is essential for survival in the host environment, where the pathogen is exposed to high levels of oxidative stress. Inhibiting RPI in M. tuberculosis could reduce the pathogen’s ability to manage oxidative damage, leading to bacterial cell death. Research into small-molecule inhibitors of RPI in M. tuberculosis has already shown promise, with several compounds demonstrating the ability to impair bacterial growth in preclinical models.[4] Similarly, protozoan parasites such as Trypanosoma brucei and Leishmania species, which cause diseases like African sleeping sickness and leishmaniasis, also rely on the PPP for nucleotide production and survival in the host. Inhibiting RPI in these pathogens could disrupt their metabolic processes, offering a novel therapeutic strategy for treating parasitic infections that are often resistant to conventional therapies.[8]
Challenges and opportunities in RPI-targeted drug development: While targeting RPI offers numerous therapeutic opportunities, several challenges must be addressed before RPI inhibitors can be translated into clinical use. One major challenge is the need for selective inhibition of RPI in disease-causing cells or pathogens without affecting the enzyme’s function in normal cells. Since RPI is conserved across species and plays an essential role in normal cellular metabolism, developing highly specific inhibitors that target only the diseased cells or pathogens is critical to avoid off-target effects and toxicity.[5]
Advances in structural biology have provided detailed insights into the active site of RPI, allowing researchers to design inhibitors that selectively bind to the enzyme in cancer cells or pathogens. Additionally, efforts to understand the differences in RPI function between healthy and diseased cells will be key to developing targeted therapies. Combination therapies that pair RPI inhibitors with other treatments, such as chemotherapy or immunotherapy, may also enhance the selectivity and efficacy of these drugs by exploiting the unique metabolic vulnerabilities of cancer cells or pathogens.[5]
Another challenge in RPI-targeted drug development is the potential for resistance. Just as cancer cells and pathogens can develop resistance to traditional therapies, they may also evolve mechanisms to bypass the inhibition of RPI. To overcome this, researchers are exploring the use of combination therapies that target multiple metabolic pathways simultaneously, reducing the likelihood of resistance and improving treatment outcomes.[7]
RPI in personalized medicine-tailoring treatments to metabolic profiles: As the field of personalized medicine continues to grow, RPI is gaining attention for its potential as a target for customized treatments based on an individual’s unique metabolic profile. Personalized medicine, also known as precision medicine, seeks to tailor treatments to the genetic, metabolic, and environmental characteristics of individual patients, offering a more targeted approach to disease management. One of the key applications of RPI in personalized medicine involves metabolic profiling, which uses advanced techniques to assess an individual’s metabolic state by analyzing the levels of various metabolites in the body. Since RPI is a critical enzyme in the PPP, changes in its activity can influence the balance of nucleotide production and redox homeostasis, both of which are crucial for maintaining cellular health. Identifying patients with altered RPI activity could help clinicians select therapies that are specifically designed to target these metabolic imbalances.[16] For example, in cancer, where the PPP is often upregulated to support rapid cell growth, patients with tumors that exhibit high RPI activity may benefit from treatments that inhibit this enzyme. Conversely, patients with metabolic disorders characterized by reduced RPI activity could be candidates for therapies that enhance the enzyme’s function, restoring the balance of nucleotide synthesis and antioxidant defenses.[14]
Genetic variants of RPI as biomarkers: In addition to metabolic profiling, genetic variants of RPI could serve as biomarkers for disease susceptibility and progression. SNPs in the RPI gene may affect enzyme activity, leading to variations in how individuals respond to oxidative stress, infections, or cancer treatments. Identifying these genetic variants through NGS technologies can provide valuable insights into a patient’s risk for developing certain diseases or their likely response to specific therapies. For instance, individuals with genetic variants that reduce RPI activity may be more susceptible to diseases that involve oxidative stress, such as neurodegenerative conditions like Alzheimer’s disease and Parkinson’s disease. Understanding the genetic basis of RPI activity can guide clinicians in developing personalized treatment plans that target specific metabolic vulnerabilities, improving patient outcomes.[8]
Moreover, identifying RPI mutations associated with rare metabolic disorders, such as RPI deficiency, can enable early diagnosis and intervention. In these cases, gene therapy or enzyme replacement therapy may be used to restore normal RPI activity, preventing the progression of metabolic imbalances and associated neurological symptoms.[9]
RPI in drug metabolism and pharmacogenomics: Another promising area of research involves the role of RPI in drug metabolism and pharmacogenomics, the study of how genetic variations affect an individual’s response to drugs. The PPP, in which RPI plays a key role, generates NADPH, a critical molecule for the detoxification of drugs and xenobiotics through the cytochrome P450 system in the liver. Variations in RPI activity could, therefore, influence a patient’s ability to metabolize certain medications, impacting drug efficacy and safety.[2] In patients with reduced RPI activity, the diminished production of NADPH may impair the liver’s ability to detoxify drugs, leading to increased toxicity or adverse drug reactions. Conversely, patients with high RPI activity may metabolize drugs more rapidly, potentially reducing their therapeutic effectiveness. By incorporating RPI activity into pharmacogenomic analyses, clinicians can better predict how patients will respond to specific medications and adjust treatment protocols accordingly.[2]
RPI in aging and age-related diseases: Aging is associated with significant changes in cellular metabolism, particularly in pathways related to oxidative stress and energy production. Ribose-5-phosphate isomerase, through its role in the pentose phosphate pathway, plays a central role in managing oxidative stress by generating NADPH, which is required for the regeneration of reduced glutathione (GSH), one of the body’s primary antioxidants. As individuals age, a decline in PPP activity, including reduced RPI function, can exacerbate oxidative damage, contributing to the progression of age-related diseases such as cardiovascular disease, neurodegeneration, and cancer.[5] Research has shown that enhancing PPP activity, including boosting RPI function, may improve redox homeostasis in aging cells, helping to counteract the effects of oxidative stress. This approach could delay the onset of age-related diseases and promote healthy aging by protecting cells from oxidative damage and maintaining proper energy metabolism. In animal models, increasing RPI activity has been linked to improved mitochondrial function, reduced oxidative damage, and increased lifespan.[19]
Additionally, therapeutic interventions aimed at enhancing RPI activity in specific tissues, such as the brain, heart, or liver, could offer a new strategy for treating age-related diseases. For example, in neurodegenerative diseases like Alzheimer’s, where oxidative stress plays a key role in disease progression, increasing RPI activity in neurons could improve antioxidant defenses and protect against neurodegeneration.[16]
Integrating RPI into Clinical Practice: As research into RPI continues to advance, its integration into clinical practice holds great promise. Personalized medicine, pharmacogenomics, and anti-aging therapies represent just a few of the potential applications of targeting RPI in human health. However, several challenges remain before these approaches can be widely implemented in the clinic. First, developing reliable diagnostic tests to measure RPI activity in patients will be crucial for translating these findings into practice. Advances in metabolomics, genetic testing, and high-throughput screening technologies are making it possible to assess RPI activity more accurately and efficiently. Standardizing these tests and ensuring their accessibility in clinical settings will be key to unlocking the full potential of RPI-targeted therapies.[13]
Second, the development of selective RPI modulators, whether as inhibitors for cancer therapy or activators for metabolic and age-related diseases, will require extensive research into the structure and function of the enzyme. Understanding the regulatory mechanisms that control RPI activity, as well as its interactions with other metabolic enzymes, will be essential for designing effective drugs with minimal off-target effects.[2]
Post-translational modifications and RPI function
Post-translational modifications (PTMs), such as phosphorylation, acetylation, and ubiquitination, are known to regulate the activity and stability of metabolic enzymes. However, little is known about how PTMs affect RPI. Future studies exploring the role of PTMs in modulating RPI’s catalytic efficiency and interaction with other enzymes in the PPP could open new avenues for therapeutic intervention. For instance, phosphorylation of RPI at specific residues might alter its activity in response to cellular stress or nutrient availability, providing a potential target for drug development.[7] Similarly, understanding how PTMs influence RPI’s stability and degradation could lead to the development of therapies that enhance or reduce its activity in specific disease contexts. In cancer, for example, promoting the degradation of RPI through ubiquitination might be a viable strategy for limiting tumor growth, while in metabolic disorders, stabilizing the enzyme could help restore normal metabolic function.
RPI’s expanding role in global health
Beyond its applications in individualized treatment, RPI holds great potential in addressing global health challenges, particularly in the context of infectious diseases and cancer. With antimicrobial resistance on the rise, targeting metabolic enzymes like RPI in pathogens offers a novel strategy for combating drug-resistant infections. Inhibitors that selectively block RPI in pathogens could weaken their metabolic defenses against oxidative stress, making them more vulnerable to immune responses or traditional therapies.[4]
Conclusion
In the context of global cancer care, RPI-targeted therapies could offer new hope for treating cancers in low- and middle-income countries, where access to cutting-edge treatments is limited. By developing affordable, metabolically-targeted therapies that exploit cancer-specific metabolic reprogramming, it may be possible to provide more accessible treatment options for patients worldwide. Furthermore, as the understanding of RPI’s role in cancer biology grows, its modulation could be incorporated into cancer prevention strategies, particularly for populations at high risk for malignancies driven by metabolic dysfunction.[14]
One of the most promising areas for future RPI research lies in the field of aging and age-related diseases. As the global population continues to age, finding ways to mitigate the effects of cellular aging and oxidative stress is a growing priority for researchers. RPI, with its central role in the production of NADPH, offers a key target for interventions aimed at reducing oxidative damage and maintaining cellular function in aging tissues.[16]
References
- Wang R, Xu X, Yao X, et al. Enhanced isomerization of rare sugars by ribose-5-phosphate isomerase A from Ochrobactrum sp. CSL1. Enzyme Microb Technol. 2021;148:109789. doi:10.1016/j.enzmictec.2021.109789 PubMed | Crossref | Google Scholar
- Stincone A, Prigione A, Cramer T, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90(3):927-963. doi:10.1111/brv.12140 PubMed | Crossref | Google Scholar
- Faria J, Loureiro I, Santarém N, et al. Disclosing the essentiality of ribose-5-phosphate isomerase B in Trypanosomatids. Sci Rep. 2016;6(1):26937. doi:10.1038/srep26937 PubMed | Crossref | Google Scholar
- Roos AK, Burgos E, Ericsson DJ, Salmon L, Mowbray SL. Competitive inhibitors of Mycobacterium tuberculosis ribose-5-phosphate isomerase B reveal new information about the reaction mechanism. J Biol Chem. 2005;280(8):6416-6422. doi:10.1074/jbc.M412018200 PubMed | Crossref | Google Scholar
- Jung CH, Hartman FC, Lu TYS, et al. D-ribose-5-phosphate isomerase from spinach: heterologous overexpression, purification, characterization, and site-directed mutagenesis of the recombinant enzyme. Arch Biochem Biophys. 2000;373(2):409-417.doi:org/10.1006/abbi.1999.1554 PubMed | Crossref | Google Scholar
- Le Moigne T, Crozet P, Lemaire SD, Henri J. High-resolution crystal structure of chloroplastic ribose-5-phosphate isomerase from Chlamydomonas reinhardtii—an enzyme involved in the photosynthetic Calvin-Benson cycle. Int J Mol Sci. 2020;21(20):7787. doi:10.3390/ijms21207787 PubMed | Crossref | Google Scholar
- Edwards TE, Abramov AB, Smith ER, et al. Structural characterization of a ribose-5-phosphate isomerase B from the pathogenic fungus Coccidioides immitis. BMC Struct Biol. 2011;11:39. doi:10.1186/1472-6807-11-39 PubMed | Crossref | Google Scholar
- Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347-354. doi: 10.1016/j.tibs.2014.06.005 PubMed | Crossref | Google Scholar
- Jung CH, Hartman FC, Lu TYS, Larimer FW. D-ribose-5-phosphate isomerase from spinach: heterologous overexpression, purification, characterization, and site-directed mutagenesis of the recombinant enzyme. Arch Biochem Biophys. 2000;373(2):409-417.doi: 10.1006/abbi.1999.1554 PubMed | Crossref | Google Scholar
- Huck JH, Verhoeven NM, Struys EA, et al. Ribose-5-phosphate isomerase deficiency: new inborn error in the pentose phosphate pathway associated with a slowly progressive leukoencephalopathy. Am J Hum Genet. 2004;74(4):745-751. doi:10.1086/383204 PubMed | Crossref | Google Scholar
- Stone VK, Kudo KY, August PM, et al. Polyols accumulated in ribose-5-phosphate isomerase deficiency increase mitochondrial superoxide production and improve antioxidant defenses in rats prefrontal cortex. Int J Dev Neurosci. 2014;37:21-25. doi:10.1016/j.ijdevneu.2014.06.009 PubMed | Crossref | Google Scholar
- Rashida Z, Laxman S. The pentose phosphate pathway and organization of metabolic networks enabling growth programs. Curr Opin Syst Biol. 2021;28:100390. doi:10.1016/j.coisb.2021.100390 Crossref | Google Scholar
- Xia N, Guo X, Guo Q, et al. Metabolic flexibilities and vulnerabilities in the pentose phosphate pathway of the zoonotic pathogen Toxoplasma gondii. PLoS Pathog. 2022;18(9) doi:10.1371/journal.ppat.1010864 PubMed | Crossref | Google Scholar
- Tenen DG, Chai L, Tan JL. Metabolic alterations and vulnerabilities in hepatocellular carcinoma. Gastroenterol Rep. 2021;9(1):1-13. doi:10.1093/gastro/goaa066 PubMed | Crossref | Google Scholar
- Ge T, Yang J, Zhou S, et al. The role of the pentose phosphate pathway in diabetes and cancer. Front Endocrinol (Lausanne). 2020;11:365. doi:10.3389/fendo.2020.00365 PubMed | Crossref | Google Scholar
- Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43(4):2466-2485. doi:10.1093/nar/gkv047 PubMed | Crossref | Google Scholar
- Schiliro C, Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 2021;10(5):1056.doi:10.3390/cells10051056 PubMed | Crossref | Google Scholar
- Alfarouk KO, Ahmed SB, Elliott RL, et al. The pentose phosphate pathway dynamics in cancer and its dependency on intracellular pH. Metabolites. 2020;10(7):285.doi:10.3390/metabo10070285 PubMed | Crossref | Google Scholar
- Segal J, Mülleder M, Krüger A, et al. Low catalytic activity is insufficient to induce disease pathology in triosephosphate isomerase deficiency. J Inherit Metab Dis. 2019;42(5):839-849. doi:10.1002/jimd.12105 PubMed | Crossref | Google Scholar
- Kitahara K, Miyazaki K. Specific inhibition of bacterial RNase T2 by helix 41 of 16S ribosomal RNA. Nat Commun. 2011;2(1):549. doi:10.1038/ncomms1553 PubMed | Crossref | Google Scholar
- Francois JM, Alkim C, Morin N. Engineering microbial pathways for production of bio-based chemicals from lignocellulosic sugars: current status and perspectives. Biotechnol Biofuels. 2020;13(1):118. doi:10.1186/s13068-020-01744-6 PubMed | Crossref | Google Scholar
- Zhang A, Liu Y, Wang F, et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22Mol Breed. 2019;39:1-10. doi:10.1007/s11032-019-0954-y PubMed | Crossref | Google Scholar
- Nielsen J, Keasling JD. Engineering cellular metabolism. Cell. 2016;164(6):1185-1197. doi:10.1016/j.cell.2016.02.004 PubMed | Crossref | Google Scholar
- Bornscheuer UT, Pohl M. Improved biocatalysts by directed evolution and rational protein design. Curr Opin Chem Biol. 2001;5(2):137-143. doi:10.1016/S1367-5931(00)00182-4 PubMed | Crossref | Google Scholar
- Sayara T, Sánchez A. Bioremediation of PAH-contaminated soils: process enhancement through composting/compost. Appl Sci. 2020;10(11):3684. doi.org/10.3390/app10113684 Crossref | Google Scholar
- Pande V, Pandey SC, Sati D, Bhatt P, Samant M. Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front Microbiol. 2022;13:824084. doi:10.3389/fmicb.2022.824084 PubMed | Crossref | Google Scholar
- Shahriari Moghadam M, Ebrahimipour G, Abtahi B, Ghassempour A, Hashtroudi MS. Biodegradation of polycyclic aromatic hydrocarbons by a bacterial consortium enriched from mangrove sediments. J Environ Health Sci Eng. 2014;12:1-9. doi:10.1186/s40201-014-0114-6 PubMed | Crossref | Google Scholar
- Broeren ML, Zijp MC, Waaijers-van der Loop SL, et al. Environmental assessment of bio-based chemicals in early-stage development: a review of methods and indicators. Biofuels Bioprod Bioref. 2017;11(4):701-718. doi:10.1002/bbb.1772 Crossref | Google Scholar
Acknowledgments
Not reported
Funding
Not reported
Author Information
Corresponding Author:
Gopi B
Department of Pharmacy
JKKMMRF’S Annai Jkk Sampoorani Ammal College of Pharmacy, Tamil Nadu, India
Email: gopi92339@gmail.com
Co-Author:
Felic S
Department of Pharmacy
JKKMMRF’S Annai Jkk Sampoorani Ammal College of Pharmacy, Tamil Nadu, India
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
Not applicable
Conflict of Interest Statement
Not reported
Guarantor
Not reported
DOI
Cite this Article
Gopi B, Felic S. Ribose-5-Phosphate Isomerase: A Critical Enzyme in Cellular Metabolism and Therapeutic Target in Disease. medtigo J Med. 2024;2(4):e30622417. doi:10.63096/medtigo30622417 Crossref









