Author Affiliations
Abstract
Sodium-benzoate (SB) is a widely used food preservative whose effects on health, especially on the memory and nervous system, are quite controversial. This study investigated the neuroprotective effects of Citrullus lanatus (C. lanatus) seed methanolic-extract on SB-induced memory/anxiety disorder in Rattusnorvegicus along with its effects on the histoarchitecture of cerebral-frontal lobe. Fifty-six Wistar rats were divided into seven groups. Group A served as control; SB-treated groups (B, D, F) were administered 200mg, 647mg and 900mg/kg body weight (bw) of SB orally respectively for 21 days; while the extract-treated group (C, E, G) received 150mg, 500mg and 800mg/kgbw of extract respectively for 14 days (orally), following SB-exposure for 21days. Neurobehavioral tests (open field and Y-maze test) were conducted to assess anxiety and memory functions, after which, the animals were sacrificed at the end of the 35-day experiment, and histological investigations were done. SB caused increased anxiety levels, impairment in memory functions, neuron degeneration, and vacuolation of varying degrees, in the frontal-lobe cortex of the treated rats, from histological findings. Administration of methanolic-extract of C. lanatus seed revitalized the degenerated neurons, ameliorated memory function impairment, but could not reduce the anxiety level. This study showed that extracts of C. lanatus seeds conferred corrective and neuroprotective effects on the treated rats. The extract served as a negative-effect-lowering agent with respect to the silent accumulative effects of SB on the neurons.
Keywords
Sodium benzoate, Methanolic extract, Citrullus lanatus, Cerebrum, Rattus norvegicus, Anxiety disorder.
Introduction
SB is generally recognized as safe, but excessive intake of these preservatives might be potentially harmful to consumers.[1] It has the chemical formula NaC7H5O2 and is a widely used food preservative. It is the sodium salt of benzoic acid and exists in this form when dissolved in water. Food preservatives like SB are substances that can prevent or delay changes caused by the action of microorganisms, enzymes, and/or physical agents, and inhibit the growth of molds, yeasts, and bacteria, found in a variety of products such as preserves, sauces, beverages, and juices.[2] Its high utilization by the food industry is due to the growing demand for chemically stable, safe, and durable foods.[3] Despite that the food and drug administration (FDA) considers the preservative SB to be safe, excessive intake of SB has induced hyperactivity in children, caused urticaria and has been significantly harmful to the deoxyribonucleic acid (DNA) according to Walczak-Nowicka ŁJ et al.[2-4]
The largest lobe in our body is the frontal lobe of the cerebral cortex (Frontal-lobe Cortex). It is situated in front of the cerebral hemispheres and performs the vital role of memory (short-term and long-term) preservation, motor coordination (speech and language), emotion regulation, and decision making.[5-7] According to Chieh-Hsin Lin et al.[8] SB may help improve cognitive performance in women with late-stage dementia. Although some other studies have shown that SB may help in the treatment of conditions like depression, pain, schizophrenia, autism spectrum disorders, and neurodegenerative diseases, on the contrary, has shown that it causes oxidative stress, impairs memory and motor coordination, and increases antisocial, anxiety-like, and depressive-like behaviors.[9-11]
C. lanatus, a fruit crop, is of herbaceous creeping plant belonging to the family Cucurbitaceae. It is mainly propagated by seeds and thrives best in warm areas. The sugar content and sweetness are the critical factors in determining the quality of many watermelon varieties. The fruit is known to be a good source of lycopene and carotenoids. It helps quench the free radicals that contribute to conditions like asthma, atherosclerosis, diabetes, colon cancer, and arthritis. It is also high in fibre and citrulline, an amino acid the body uses to make arginine.[12] The seeds are often discarded after the fruit is eaten. C. lanatus seeds are known to be highly nutritional; they are rich sources of protein, vitamin B varieties, minerals (such as magnesium, potassium, phosphorus, sodium, iron, zinc, manganese, and copper), and fat, among others, as well as phytochemicals.[13]
The seeds of C. lanatus have been shown to have a wide range of pharmacological and biological activities, which include antimicrobial, antioxidant, anti-plasmodial, anti-inflammatory, anti-prostatic hyperplasia activity, antigiardial activity, analgesic properties, antisecretory, antidiabetic, laxative, antiulcerogenic, and hepatoprotective activities. The seed is used in the treatment of urinary tract infections, bedwetting, dropsy and renal stones, alcohol poisoning, hypertension, diabetes, diarrhea, and gonorrhea.[14] Watermelon varieties are said to contain high amounts of antioxidants, including citrulline and lycopene. C. lanatus seeds contain an antioxidant known as cucurbocitrin, which is extracted and used in lowering blood pressure and improving kidney function.[12]
We therefore hypothesized that the methanolic extract of C. lanatus seed could demonstrate protective effects on the damage induced by SB in the cerebral cortex of the frontal lobe in Wistar rats.
Methodology
Plant procurement and preparation of extract: The watermelon (C. lanatus) used for this study was purchased from the Oje market in Ibadan. The seeds were removed from the pulp with the aid of a sterilized knife, the seeds were cleaned, washed, air dried, and carefully ground into a coarse form using a mechanical blender. The method of extraction employed was described by Abdu, H et al.[13] The blended watermelon seed (945g) was macerated in 1900 ml of methanol for 72 hours. The macerate was passed through whatman No. 4 filter paper. The filtrate was concentrated in a rotatory evaporator and dried in a boiling water bath. The extract yield for the methanolic extract was calculated as:
Mass of crude plant
The extract yield was 14.3%
Lethal dose (LD50): Shimaa Mohammad Yousef et al[15] reported LD50 value of SB in rats as 3140 mg/kg body weight, while Damilola et al[16] concluded that administration of up to 5000 mg/kg body weight of methanolic extract of C. lanatus seeds caused no death in the two stages of the test. Thus, the LD50 of methanolic extract in mice was estimated to be greater than or equal to 5000 mg/kg body weight.
Experimental design: The ethical committee of the department of anatomy, Olabisi Onabanjo University, Sagamu campus, gave approval for this study. The rats were divided into 7 groups (A-G), consisting of 8 rats in each group. The weights of the rats at the start of the experiment (in grams), the dosage of SB and C. lanatus seed extract (CLSE) administered are shown in Table 1.
| Groups | N | Average weight | Administration | Duration |
| A (Control) | 8 | 170.2g ± 24.9 | Normal saline | 5 weeks |
| B | 8 | 197.4g ± 36.2 | 200mg/kg SB | 3 weeks |
| C | 8 | 185.6g ± 32.8 | 200mg/kg SB + 150mg/kg CLSE | 5 weeks |
| D | 8 | 181.5g ± 30.1 | 647mg/kg SB | 3 weeks |
| E | 8 | 186.8g ± 29.1 | 647mg/kg SB + 500mg/kg CLSE | 5 weeks |
| F | 8 | 154.8g ± 25.6 | 900mg/kg SB | 3 weeks |
| G | 8 | 150g ± 26.2 | 900mg/kg SB + 800mg/kg CLSE | 5 weeks |
Table 1: Table of administration (experimental design)
Behavioral test: The rats were tested for spatial short-term/working memory, using the Y-maze test, and locomotion and anxiety, using the open field test.
Y-maze test: The Y-maze test procedure was described by Lisa Blackmer-Raynolds et al.[17] The Y-maze is a quick and easy way to evaluate a rodent’s spatial working memory by measuring their ability to effectively explore a Y-shaped maze. Rodents typically prefer to explore novel arms of the maze, rather than returning to arms they have previously visited. As such, rodents with intact working memory will alternate between all arms of the maze before returning to one they have visited previously.

Figure 1: Showing the Y-maze test apparatus used
Open field test: The Open-field test procedure was described by CastanheiraLígia et al[18] and Göntér K et al.[19] The open field apparatus was constructed with white plywood and measured 72 x 72cm with 36 cm walls. One of the walls was clear Plexiglas, So rats could be visible in the apparatus. Blue lines were drawn on the floor with a marker and were visible through the clear Plexiglas wall. The lines divided the floor into sixteen 18 x 18 cm squares. A central square (18 cm x 18 cm – red) was drawn in the middle of the open field.

Figure 2: Showing the open field test apparatus used
Results
Differential body weight results:
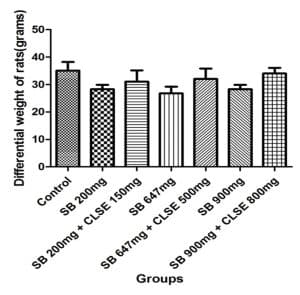
Figure 3: It is a graphical representation of the body weights of the treated rat following the treatment period. The graph revealed there was no significant statistical difference across the groups (P <0.05 ± Standard error of the mean [SEM], using one-way analysis of variance [ANOVA])
Y-Maze test results: Figure 4 gives a representation of the spontaneous alternation percentage (SAP) following 5 minutes of exploration in the y-maze, and the alternate arm return (AAR) of the rats exposed to SB and CLSE for 35 days. SAP assessed the memory function and memory impairment in the rats.
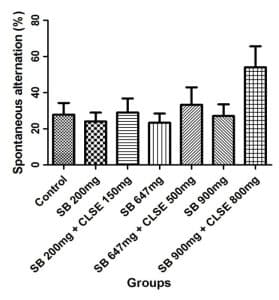
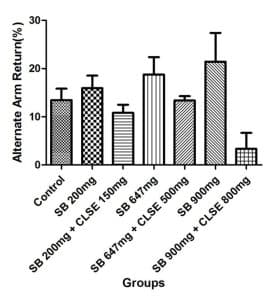
Figure 4: Graphs of SAP and AAR after 35 days of treatment, showing assessment of the memory function and memory impairment in the treated rats
Each bar represents Mean±SEM with (n=8). For SAP, the groups administered sodium-benzoate (B, D, and F) recorded low SAP% when compared to the control group (group A) and other groups treated with the C. lanatus seed extract (C, E, and G), after their exposure to SB. For AAR, the groups administered SB (B, D, and F) recorded high AAR% % when compared to the control group (group A) and when compared with groups treated with the C. lanatus seed extract (groups C, E, and G) after their exposure to SB.
Open field test results: Figure 5 shows an assessment of locomotory activities and anxiety, using the frequency of line crossing of rats when exposed to SB and C. lanatus seed extract.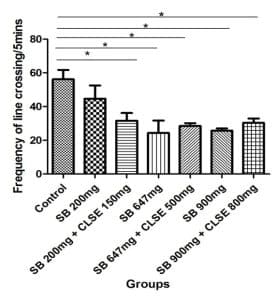
Figure 5: Showing the frequency of line crossing within the open field maze following 5 minutes of exploration. A significant decrease in locomotion was observed across all groups when compared to the control group (at p < 0.05 ±SEM, ANOVA)
Histological results:
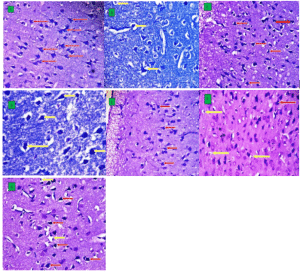
Figure 6: Kluver-Barrera (KB) Staining
Photomicrograph section of cerebral frontal lobe (A-G) shows the histoarchitecture and cellular organization across the experimental groups (KB X400). Legend: Normal pyramidal neurons (red arrows); Aberrant cells: degenerated, vacuolated, and pyknotic cells (yellow arrow).
Group A presents with an abundance of normal pyramidal cells, having centrally located nuclei; Group B, D and F showed degenerating pyramidal neurons, surrounded with vacuolated cells and poor cellular integrity, which can be attributed to SB administration; while Group C, E and G showed an abundance of healthy and normal cells, with varying degrees of vacuolated and pyknotic cells, showing ongoing recovery, mediated by C. lanatus seed extract administration.
Discussion
This present study determines the effects of methanolic-extract of C. lanatus seed on body weights of rats, neurobehavioral attributes (memory impairment and anxiety disorder), and the histoarchitecture of cerebral-frontal lobe following damage induced by SB. This is with a view to determining the neuroprotective potential of C. lanatus seed.
According to the differential body weight graph, the control group (A) and extract-treated groups (C, E, G) have a body weight increase compared to the SB-treated groups (B, D, F) as seen in Figure 3. However, they were insignificant, suggesting that despite its toxicity, SB did not cause overall sufficient organ or tissue necrosis, which might have led to a significant reduction in body weight of rats in the present study. This agrees with the work that revealed that SB does not have a significant impact on body weight.[20]
The neurobehavioral test analyses (Y-maze) were used to evaluate spatial memory and learn in experimental rats. A higher percentage of SAP indicates normal memory function, and a higher percentage of AAR indicates impairment in memory function and learning. SAP and AAR of rats as shown in Figure 4 revealed that SB-treated groups (groups B, D, and F) had low SAP and high AAR, indicating impairment in the memory functions of rats in groups treated with SB. However, rats in groups treated with the extract after exposure to SB (groups C, E, and G) had high SAP and low AAR, indicating that the impairment in memory functions was corrected in groups treated with the extract. Khoshnoud et al[21] reported that SB significantly impaired memory and motor coordination after oral administration of different concentrations of SB for 4 weeks, while Noorafshan et al[22] reported that the performance of the SB-treated rats was impaired in the elevated plus maze, an indicator of anxiety. Their riding time in fixed and accelerating speed rotarods decreased, indicating motor impairment.
The open field analysis was used to assess measures of locomotion, exploration, and anxiety[18]. The number of line crosses was used as a measure of locomotor activity, but is also a measure of exploration and anxiety. A high frequency of these behaviors indicates increased locomotion and exploration and/or a lower level of anxiety and vice versa. Statistical analysis of the open field as shown in Figure 5 revealed a significant decrease at p < 0.05 in locomotion and exploration of rats in all groups when compared to the control group. This shows that SB at 200mg, 647mg, and 900mg/kgbw increased anxiety level and reduced movement of rats. This also suggests that the methanolic extract of C. lanatus could not reduce the high anxiety level induced by SB. This result agrees with that of Walczak-Nowicka ŁJ et al[2] and Noorafshan et al[22] which concluded 200mg/kg/day induces anxiety in the experimented rats.
Different mechanisms can be considered by which SB can influence motor impairment and anxiety. Metabolism of benzoate in the liver is performed by conjugation with glycine. Yet, using glycine in the detoxification of benzoate results in a reduction of the glycine level in the body, which can affect the metabolic process in which glycine is involved. It has also been reported that a decrease in glycine leads to anxiety.[2]
Another mechanism that might be responsible for impairment and anxiety is zinc deficiency. SB can induce changes in the brain that significantly reduce the zinc level in the brain. This will eventually lead to behavioral changes in mice. Zinc deficiency has also been reported to be associated with cognitive and motor function impairment, depression, anxiety, and attention deficit hyperactivity disorder (ADHD) symptoms.[23] Study by Akintoye et al[10] revealed that Zinc supplements caused a remarkable improvement in memory, reversing anxiety-like behaviour in SB-induced neurotoxicity
Histological findings, as expressed through Kluver-Barrera special staining, revealed degenerating neurons, while some neurons appeared swollen in the SB-treated groups, indicating damage caused by SB. However, normal neurons and normal layer are seen in extract-treated groups (C, E, G) after their exposure to SB, as seen in plates C, E, and G. Alameen et al[24] reported that histopathological examination of the brain of rats treated with SB showed mild degenerative and necrotic changes. Mild congestion was found in the brains of rats in acute toxicity. Noorafshan et al[23] also reported that SB exposure with or without ascorbic acid treatment could alter the cerebellum.
The consequence of degeneration of frontal cerebral cortex neurons includes the inability of the animal or human to perform executive functions such as self-control, planning, reasoning, attention, decision making, judgments, overall control of motor function, and abstract thought.[25] In rats, the acute implication would include a reduction in locomotor and exploration abilities, buttressed by the results of the open field maze test of this study, which showed that locomotor and exploratory abilities decreased in SB-treated rats. There could also be upper motor neuron lesion manifestations since the corticospinal tract and some other important corticofugal projection fibres are associated with the frontal cortex, although this was not demonstrated in this experiment[25]
Conclusion
In conclusion, SB increased the anxiety level & induced impairment in memory functions of rats and caused degeneration, degenerating, and swollen neurons in the cerebral frontal cortex from histological findings. Administration of methanolic extract of C. lanatus seed revitalized the degeneration in neurons in the cerebral frontal cortex, corrected the impairment in memory function, but could not reduce the anxiety level.
References
- Asejeje FO, Ajayi BO, Abiola MA, et al. Sodium benzoate induces neurobehavioral deficits and brain oxido-inflammatory stress in male Wistar rats: Ameliorative role of ascorbic acid. J Biochem Mol Toxicol. 2022;36(5):e23010. doi:10.1002/jbt.23010 PubMed | Crossref | Google Scholar
- Walczak-Nowicka ŁJ, Herbet M. Sodium benzoate—Harmfulness and potential use in therapies for disorders related to the nervous system: A review. Nutrients. 2022;14(7):1497. doi:10.3390/nu14071497
PubMed | Crossref | Google Scholar - Thomas OE, Adegoke OA. Toxicity of food colours and additives: A review. Afr J Biotechnol. 2015;9(36):900-914.4B78EBE55367 (academicjournals.org)
- Food and Drug Administration. Food and beverages. 2017. FDA Requirements for Food & Beverage | Registrar Corp
- Neulinger K, Oram J, Tinson H, O’Gorman J, Shum DHK. Prospective memory and frontal lobe function. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016;23(2):171-183. doi:10.1080/13825585.2015.1066734 PubMed | Crossref | Google Scholar
- Flinker A, Korzeniewska A, Shestyuk AY, et al. Redefining the role of Broca’s area in speech. Proc Natl Acad Sci USA. 2015;112(9):2871-2875. doi:10.1073/pnas.1418462112 PubMed | Crossref | Google Scholar
- Saladin KS. Human Anatomy. 3rd ed. McGraw-Hill; 2011. 9781260210262.pdf
- Lin CH, Chen PK, Wang SH, Lane HY. Effect of sodium benzoate on cognitive function among patients with behavioral and psychological symptoms of dementia: Secondary analysis of a randomized clinical trial. JAMA Netw Open. 2021;4(4):e216156. doi:10.1001/jamanetworkopen.2021.6156 PubMed | Crossref | Google Scholar
- Gaur H, Purushothaman S, Pullaguri N, Bhargava Y, Bhargava A. Sodium benzoate induced developmental defects, oxidative stress and anxiety-like behaviour in zebrafish larva. Biochem Biophys Res Commun. 2018;502(3):364-369. doi:10.1016/j.bbrc.2018.05.120 PubMed | Crossref | Google Scholar
- Akintoye OO, Ajibare AJ, Folawiyo MA, et al. Zinc supplement reverses short-term memory deficit in sodium benzoate-induced neurotoxicity in male Wistar rats by enhancing anti-oxidative capacity via Nrf2 up-regulation. Behav Brain Res. 2023;437:114163. doi:10.1016/j.bbr.2022.114163 PubMed | Crossref | Google Scholar
- Bellezza I, Tucci A, Galli F, et al. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J Nutr Biochem. 2012;23(12):1583-1591. doi:10.1016/j.jnutbio.2011.10.008 PubMed | Crossref |
Google Scholar - Gbolahan AO, Clara TF. Review of studies published on the medicinal importance of different parts of Citrullus lanatus in the last ten years. J Biol Res Biotechnol. 2021;19(2):1372-1387. doi:10.4314/brb.v19i2.8 Crossref |
Google Scholar - Abdu H, Ahmad FBH, Adamu AU, Sai’du AA. Phytochemical screening, proximal analysis, and anti-ulcer activity of methanolic seed extract of Citrullus lanatus. Afr J Agric Sci Food Res. 2023;13(1):42-50. Phytochemical Screening, Proximate Analysis and Anti-ulcer Activity of Methanolic Seed Extract of Citrullus lanatus | African Journal of Agricultural Science and Food Research
- Odo C, Okeke U, Akuaden S, et al. Haematinic and haematopoietic potentials of methanolic extract of Citrullus lanatus (watermelon) seeds in experimental rats. J Pharm Allied Med. 2023;1:71-77. doi:10.58985/jpam.2023.v01i02.09 Crossref
- Youssef SM, Erfan H, Zayed MA, El-Sayed K. Assessment of the effect of concomitant use of sodium benzoate and fructose on the liver structure and function in young albino rats. Med J Cairo Univ. 2021;89(2):761-767. doi:21608/mjcu.2021.168010 Crossref | Google Scholar
- Damilola AO, Adekunle AA. Effect of methanolic extract of Citrullus lanatus seed on lipid profile and oxidative stress in acetaminophen-intoxicated rats. Adv Biomed Pharm Sci. 2016;3(2):87-93. doi:10.19046/abp.v03i02.02 Crossref
- Blackmer-Raynolds L, Krout IN, Sampson T. Y-Maze protocol. io. 2024. doi:10.17504/protocols.io.xyz789 Crossref
- Castanheira L, Ferreira FM, Ana TS, Telles-Correia D. Anxiety assessment in pre-clinical tests and in clinical trials: A critical review. Curr Top Med Chem. 2018;18(19):1730-1742. doi:10.2174/1568026618666180717165636 PubMed | Crossref | Google Scholar
- Göntér K, Dombi Á, Kormos V, et al. Examination of the effect of dimethyl trisulfide in an acute stress mouse model with the potential involvement of the TRPA1 ion channel. Int J Mol Sci. 2024;25(14):7701. doi:10.3390/ijms25147701 PubMed | Crossref | Google Scholar
- Cetin S, Yagut E, Ruslan B, et al. Effect of sodium benzoate on DNA breakage, micronucleus formation and mitotic index in peripheral blood of pregnant rats and their newborns. Biotechnol Biotechnol Equip. 2016;30(6):1179-1183. doi:10.1080/13102818.2016.1224979 Crossref | Google Scholar
- Khoshnoud MJ, Siavashpour A, Bakhshizadeh M, Rashedinia M. Effects of sodium benzoate, a commonly used food preservative, on learning, memory, and oxidative stress in the brain of mice. J Biochem Mol Toxicol. 2017;31(8):e22022. doi:10.1002/jbt.22022 PubMed | Crossref | Google Scholar
- Noorafshan A, Erfanizadeh M, Karbalay-Doust S. Sodium benzoate; a food preservative, induces anxiety and motor impairment in rats. Neurosciences (Riyadh). 2014;19(1):24-28. Sodium benzoate, a food preservative, induces anxiety and motor impairment in rats
- Noorafshan A, Erfanizadeh M, Karbalay-Doust S. Stereological studies of the effects of sodium benzoate or ascorbic acid on rats’ cerebellum. Saudi Med J. 2014;35(12):1494-1500. Stereological studies of the effects of sodium benzoate or ascorbic acid on rats’ cerebellum – PMC
- Alameen S, Jirjees E, Tawfeeq F. Effect of sodium benzoate on some biochemical, physiological and histopathological aspects in adult male rats. Iraqi J Vet Sci. 2022;36:267-272. (PDF) Effect of sodium benzoate on some biochemical, physiological and histopathological aspects in adult male rats
- Owoeye O, Akinbami RO, Thomas MA. Neuroprotective potential of Citrullus lanatus seed extract (CLSE) and vitamin E against mercury chloride intoxication in male rat brain. Afr J Biomed Res. 2018;21:43-49. Neuroprotective Potential of Citrullus lanatus Seed Extract and Vitamin E Against Mercury Chloride Intoxication in Male Rat Brain | Semantic Scholar
Acknowledgments
The authors would like to acknowledge Dr P. D Shallie, Dr Adesanya Olamide, and Ifeoluwa Grace for their technical support.
Funding
This research was self-funded by the authors with no external financial support or grant.
Author Information
Corresponding Author:
Banjo Olutayo
Department of Anatomy
Olabisi Onabanjo university, Nigeria
Email: banjosamson4real@gmail.com
Co-Authors:
Akpan Bassey
Department of Anatomy
Bowen University, Nigeria
Adelakin Adeola
Department of Anatomy
Babcock University, Nigeria
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Conflict of Interest Statement
The authors declare no conflict of interest.
Guarantor
None
DOI
Cite this Article
Banjo SO, Akpan HB, Adelakin LA. Methanolic Extract of Citrullus Lanatus Seed Ameliorates Sodium Benzoate-Induced Memory Impairment, Anxiety Disorder, and Histoarchitectural Disruptions in Rattus Norvegicus Frontal-Lobe Cortex. medtigo J Neurol Psychiatry. 2024;1(1):e3084111. doi:10.63096/medtigo3084111 Crossref









