Author Affiliations
Abstract
Background: Although glioblastoma is the most common malignant primary brain tumor, it rarely presents in the intraventricular region(s) of the brain.
Case presentation: We report a unique case of intraventricular glioblastoma in a 62-year-old Hispanic female, distinguished by her lack of substantial tumor recurrence.
Conclusion: Our report suggests the importance of understanding the management and treatment of intraventricular glioblastoma in neurosurgical patients. In addition, our study emphasizes the importance of treating intraventricular glioblastoma in patients, as survival rates and post-operative quality of life can result in improved function.
Keywords
Glioblastoma, Intraventricular region, Lateral ventricle, Survival rate, Post-operative life.
Introduction
Glioblastomas are high-grade astrocytomas and the most common primary malignant brain tumors of the central nervous system (CNS), encompassing a quarter of all adult intracranial tumors and half of all glial tumors.[1-3] The aggressive nature of the tumor results in a median survival of approximately 14.6 months for adults treated with concurrent chemotherapy and radiation therapy.[4] Although glioblastoma can arise at any location in the CNS, intraventricular regions are relatively rare.[5]
The subventricular zone (SVZ) is defined as a region located on the lateral wall of the lateral ventricle, where neural stem cells are more vulnerable to tumor proliferation compared to cortical regions. Studies have shown a worse prognosis with subependymal-spreading tumors, as opposed to non-subependymal-spreading tumors.[6,7] Good prognostic factors include young age, high Karnofsky Performance Status (KPS), high mini-mental status examination score, O6-methylguanine methyltransferase promoter methylation, and resection of >98% of the tumor.[8] There are only 16 reported adult intraventricular glioblastoma multiforme (GBM) cases to our knowledge.[1, 2, 5, 9-13]
In this case report, we describe a case of intraventricular GBM that is notable due to her prolonged survival of 20 months and lack of significant tumor mass recurrence.
Case Presentation
History and Examination: A 62-year-old Hispanic female was admitted to the neurosurgery unit after presenting to the emergency department with complaints of headaches and reported behavioral changes. On the initial presentation, the patient was alert and oriented to person and place. She moved all extremities equally but had an unsteady gait. Cranial nerves II through XII were grossly intact. The remainder of her neurological exam did not include any focal deficits. Past surgical history was remarkable for colon cancer in remission after colectomy, cholecystectomy, hysterectomy, and bilateral knee arthroscopy repair x 3. Past medical history included osteoporosis, hyperlipidemia, steroid-induced hyperglycemia, and chronic anemia. She did not report any tobacco or alcohol use.
Imaging: A non-contrast computed tomography (CT) identified an intraventricular soft tissue mass centered at the anterior aspect of the septum pellucidum covering the foramen of Monro bilaterally and extending to the body of the corpus callosum (Figure 1).
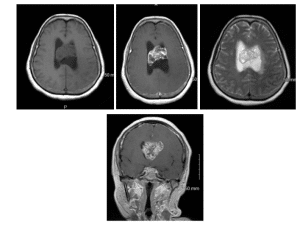
Figure 1: Non-contrast axial CT images of 62 y/o female with intraventricular mass at the anterior aspect of the septum pellucidum covering the foramen of Monro bilaterally and extended to the body of the corpus callosum
The left lateral ventricle was moderately enlarged. Multiplanar magnetic resonance imaging was significant for a large 4.6 x 4.3 x 2.9 cm mass extending from the foramen of Monro, occupying the entire anterior aspect of the lateral ventricles (Figure 2). CT of the chest, abdomen, and pelvis was negative for any other masses. The patient had some complaints about back pain. The entire neuroaxis was scanned, as a primary intraventricular tumor was also in the differential.
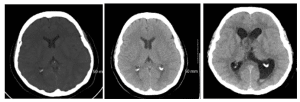
Figure 2: (A) Non-contrast axial T1 MRI (B) Axial T1 MRI with contrast (C) T2 axial MRI without contrast
Case Management
Operation and pathological findings: The patient underwent a right frontal craniotomy. An interhemispheric transcallosal approach was used to reach the tumor. Frameless stereotactic navigation, microscope, and microsurgical techniques were employed. The dissection corridor was along the right side of the falx, and the right hemisphere was positioned downward to allow gravity to assist with retraction. The tumor was encountered upon the corpus callosotomy, and tumor dissection led to entry into the bilateral lateral ventricles. The mass was sub-totally resected with a small residual left on the side of the ventricle. The significant retraction required to reach this last piece of tumor, in addition to its adherence to the ventricular wall, weighed in our decision to leave this small residual. An external ventricular drain (EVD) was placed under direct visualization. The patient had unchanged Somatosensory Evoked Potentials (SEEP), continuous electroencephalogram (EEG) throughout the case. Post-operatively, the patient awoke with a 1/5 left hemiparesis. The tumor was found to be a high-grade IV intraventricular glioblastoma with no O6-methylguanine-DNA-methyltransferase (MGMT) gene methylation. Pathology immunohistochemical stains included neurofilament stain highlighting infiltrative tumor edges, strong glial fibrillary acidic protein (GFAP) reactivity, and nonspecific positive synaptophysin. Numerous mitotic figures were consistent with malignancy, where arrangements were in conspicuous perivascular, focal fascicular, palisading arrangements around areas of necrosis with myxoid stromal-type change and focal diffuse patterns. Proliferation index MIB-1 (Ki67) was elevated at approximately 35% (Figure 3). Prior to discharge, the patient had placement of a ventricular peritoneal shunt, as the ventriculostomy placed at surgery could not be weaned.
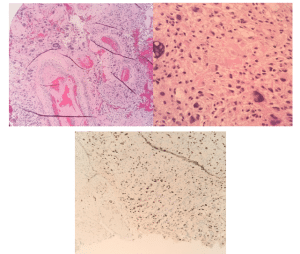
Figure 3: Histopathologic examination of solid glial neoplasm
(A) Bizarre nuclear features and vascular proliferation (10X magnification)
(B) Broad coagulative type necrosis and focal areas of cellular necrosis associated with small cell and gemistocytic change. Bizarre nuclear features and necrosis (40X magnification)
(C) Ki-67 (MIB1) immunohistochemical stain (10X magnification) showing approximately 35% staining in areas
Post-operative course to time of death: Her initial outpatient visit, one month after discharge from the hospital demonstrated improvement in muscle strength from 2/5 to 3/5 in the left upper extremity (LUE), 1/5 to 2/5 strength in the left lower extremity (LLE), and consistent 5/5 strength in right upper and lower extremities. The sensation to light touch was intact throughout, and the patient rated her pain a 0 on a scale of 10. Cranial nerves II through XII were all intact throughout, with no upward gaze palsy. She was alert and oriented with the ability to articulate her speech. As she continued her post-operative care, her hemiparesis improved to 4/5, and she regained the ability to walk with a cane.
Two weeks after her surgery, she started chemotherapy and radiation therapy regimen. Chemotherapy included 120 mg PO daily of temodar (temozolomide) for one month. This was then followed by metronomic dosing of temodar at 80 mg for 10 months, due to the patient’s tolerance. Avastin (bevacizumab) was started for the prevention of tumor recurrence and disease progression after completion of metronomic temodar.
Radiation therapy consisting of 6000 cGy was administered concurrently with this chemotherapy. Since her disease was a rare intraventricular site, inclusion of the cerebrospinal fluid (CSF), similar to the approach for carcinomatous meningitis, became a consideration. This was used for the initial approach after an extensive literature search and discussion with experts. Treatment began with a 2-field approach to the whole brain and upper cervical spine to C2, to 3600 cGy. Then, a 10-intensity modulated radiation therapy (IMRT) arrangement was used to complete the 6000 cGy (Figure 4). Before treatment, each day, image-guided radiation therapy (IGRT) cone beam analysis was used to ensure precise treatment setup.
The first magnetic resonance imaging (MRI) detected recurrence/progression of the tumor 4 months after completion of the initial course of therapy. This was occurring at the site of subtotal resection along the ventricular wall, and stereotactic radiosurgery (SRS) was planned with personal participation by the neurosurgeon to ensure that the correct treatment volumes were established. Treatment was administered using a 19-field SRS plan delivering 1800 cGy as a single fraction. The patient responded well to treatment with the first arrest, then regression of the residual mass. She remained relatively stable from 4 to 20 months, without signs of disease progression on MRI. Twenty months post-craniotomy, the patient’s performance scale began to deteriorate significantly without signs of tumor recurrence. She was ultimately placed in hospice care and expired 20 months after her diagnosis.
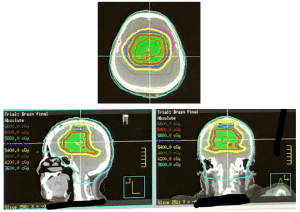
Figure 4: IMRT to localize the treatment of the tumor
Discussion
Intraventricular tumors are very rare and comprise less than 2% of all intracranial tumors.[14] The most common presentation generally occurs after the tumor grows significantly, includes signs of hydrocephalus and increased intracranial pressure, as the neoplasm can obstruct CSF movement and may require further intervention via a ventriculoperitoneal (VP) shunt.[15] A majority of the case reports describe the location of the tumor to be in the lateral ventricle or the body of the corpus callosum. The reports do not describe any CSF dissemination where the tumor metastasizes to the spine via CSF. Various transcortical approaches were reported in case reports to respect the intraventricular tumors. A majority of them require post-operative radiation and chemotherapy, therefore gross total resection is generally not completely achievable. The average age of survival from the case reports was found to be 14.75 months, which is comparable to the median survival age for parenchymal GBMs. Our patient survived for 20 months post-procedure. Some case reports did not include an average age of survival period: one report mentions a patient dying after 1 month; three do not report any time frame; and one mentioned 3 patients surviving at least two years post-surgery. Furthermore, three patients required ventriculoperitoneal shunts due to acute hydrocephalus. The characteristics of common intraventricular tumors were summarized in Table 1.[16]
| Type | Most common age group | Most common region | Typical findings |
| Choroid plexus tumors | Children <10-Year-old (yo) | Lateral or fourth ventricles | Hyperdense on CT, lobulated, enhancing |
| Meningioma | 40-60 yo, females > males | Lateral ventricle | Hyperdense on CT, calcification (50%), enhancing |
| Ependymoma | Adults and children (1/3 in <3 yo) | Fourth ventricle | Heterogenous, hemorrhage, necrosis, cyst, calcification |
| Subependymoma | 40-60 yo | Lateral ventricle or fourth ventricle | Poor enhancement, calcification (30%) |
| Subependymal giant cell astrocytoma | 0-20 yo | Foramen of Monroe | Enhancing |
| Central neurocytoma | 20-40 yo | Inferior septum pellucidum and anterior lateral ventricle | Lobulated, enhancing |
Table 1: Summarizes characteristics of common intraventricular tumors.
Although GBMs are the most common primary brain tumors, intraventricular GBMs occur rather infrequently. Most GBMs happen in those who are 55-74 years of age and are more common in men.[17] The annual incidence in the United States (US) is approximately 3 in 100,000 people and accounts for 25% of all malignant nervous system neoplasms.[17] A PubMed search was conducted using the following keywords: “intraventricular glioblastoma multiforme” and “intraventricular GBM”. The search found only 16 adult known events of intraventricular GBMs to date.[1,2,5,9-13]
Survival rates in GBM patients are very low: less than 30% at 1 year, 5% at 3 years, 3% at 5 years.[17] One clinical trial reported a 5-year survival rate of 9.8% in patients who were treated with radiation therapy and temozolomide.[18] The radiation therapy oncology group (RTOG) prognostic classification helps estimate median survival. RTOG class V can be used for our patients (i.e. patients older than 50 years of age with of the following: KPS of 70-100 and resection with neurologic deficits, KPS 70-100 and only biopsy followed by at least 54.4 Gy of radiation therapy, or KPS < 70 and no neurologic deficits).[19,20] Median survival for the RTOG V group is 8.9-10.7 months. Tumor genetic expression has the potential to affect prognosis.
The MGMT gene is involved in deoxyribonucleic acid (DNA) repair and reversal of any DNA damage, and when the promoter region of MGMT is hypermethylated, tumor cells more sensitive to treatment with alkylating agents, therefore a survival mechanism.[21] GBMs are pathologically poorly differentiated neoplastic astrocytes that consist of a diffuse infiltrative growth pattern, making it difficult for neurosurgeons to completely resect the tumor.[17] This patient’s past chemotherapy and radiation exposure for the diagnosis and treatment of colon cancer could have influenced tumor growth. Although it is not known if this is a definitive cause for this patient’s GBM, it can potentially play a role in contracting future malignancies. Our patient survived for a total of 20 months post-surgical intervention, which is more than the current average lifespan of GBM; however, our literature review did find a few instances where the survival rate was similar or slightly more. Of note, some studies did not report full patient follow-up up to the time of death. This could be attributed to the patient’s death and may have an impact on interpreting an accurate post-operative lifespan.
Our patient had an increased proliferation index with several risk factors, including therapeutic radiation exposure and an increased KPS score. Some challenges involved with tumor removal include safely dissecting its external surface from surrounding neural structures and obstructing the ventricle cavity.[9] Even with total tumor resection, minuscule remnants of tumor are frequently left behind.[9] Typical GBM standard of care includes maximal tumor resection followed by adjuvant chemoradiation therapy. Stupp et al. advise the use of the chemotherapeutic agent, Temodar, given at 75 mg/m2 daily for 6 to 7 weeks, and 150-200 mg/m2 given for 5 days every 28 days for approximately 6 cycles.[18] Although the incidence of GBM in a colon cancer survivor patient is unknown, the effects of radiation and chemotherapy should be considered and revisited when determining possible causes. Changes in neurological examination should be followed up with re-evaluation of the patient, and additional surgical procedures should be considered with risks and benefits in mind. Furthermore, baseline neuroimaging should be included in the initial assessment, and additional imaging should regularly be conducted to monitor any future tumor growth.
Conclusion
This case report describes a neurosurgeon’s experience with intraventricular glioblastoma in a patient with a history of colon cancer. Based on our experience and review of the literature, this patient had no signs of gross tumor recurrence and functionally benefits from her surgery and treatment. Although intraventricular tumors are rare, gross total resection can safely be performed with promising patient functionality.
References
- Lee TT, Manzano GR. Third ventricular glioblastoma multiforme: case report. Neurosurg Rev. 1997;20(4):291-294. doi:10.1007/BF01105903 PubMed | Crossref | Google Scholar
- Secer HI, Dinc C, Anik I, Duz B, Gonul E. Glioblastoma multiforme of the lateral ventricle: report of nine cases.
Br J Neurosurg. 2008;22(3):398-401. doi:10.1080/02688690701867254 PubMed | Crossref | Google Scholar - Guibaud L, Champion F, Buenerd A, Pelizzari M, Bourgeois J, Pracros JP. Fetal intraventricular glioblastoma: ultrasonographic, magnetic resonance imaging, and pathologic findings. J Ultrasound Med. 1997;16(4):285-288. doi:10.7863/jum.1997.16.4.285 PubMed | Crossref | Google Scholar
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi:10.1056/NEJMoa043330 PubMed | Crossref | Google Scholar
- Kim YJ, Lee SK, Cho MK, Kim YJ. Intraventricular glioblastoma multiforme with previous history of intracerebral hemorrhage: a case report. J Korean Neurosurg Soc. 2008;44(6):405-408. doi:10.3340/jkns.2008.44.6.405
PubMed | Crossref | Google Scholar - Savarese G, Kishi T, Vardeny O, et al. Heart Failure Drug Treatment-Inertia, Titration, and Discontinuation: A Multinational Observational Study (EVOLUTION HF). JACC Heart Fail. 2023;11(1):1-14. doi:10.1016/j.jchf.2022.08.009 PubMed | Crossref | Google Scholar
- Parsa AT, Wachhorst S, Lamborn KR, et al. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J Neurosurg. 2005;102(4):622-628. doi:10.3171/jns.2005.102.4.0622
PubMed | Crossref | Google Scholar - Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18(8):1061-1083. doi:10.1517/13543780903052764
PubMed | Crossref | Google Scholar - Park P, Choksi VR, Gala VC, Kaza AR, Murphy HS, Ramnath S. Well-circumscribed, minimally enhancing glioblastoma multiforme of the trigone: a case report and review of the literature. AJNR Am J Neuroradiol. 2005;26(6):1475-8.
Well-circumscribed, minimally enhancing glioblastoma multiforme of the trigone: a case report and review of the literature - Prieto R, Pascual JM, Roda JM. Third ventricle glioblastoma. Case report and review of literature. Clin Neurol Neurosurg. 2006;108(2):199-204. doi:10.1016/j.clineuro.2004.12.012 PubMed | Crossref | Google Scholar
- Sarikafa Y, Akçakaya MO, Sarikafa S, Ozkaya F, Akdemir O, Celik SE. Intraventricular glioblastoma multiforme: Case report. Neurocirugia (Astur). 2015;26(3):147-150. doi:10.1016/j.neucir.2014.09.001 PubMed | Crossref | Google Scholar
- Patnaik A, Mishra SS, Senapati SB. Intraventricular glioblastoma multiforme mimicking meningioma and review of the literature. Asian J Neurosurg. 2017;12(1):75-77. doi:10.4103/1793-5482.145104 PubMed | Crossref | Google Scholar
- Asha MJ, Tansey RJ, Gan YC. ‘Goose bumps’ as presenting feature of intraventricular glioblastoma multiforme.
Br J Neurosurg. 2014;28(2):276-277. doi:10.3109/02688697.2013.817530 PubMed | Crossref | Google Scholar - Gökalp HZ, Yüceer N, Arasil E, et al. Tumours of the lateral ventricle. A retrospective review of 112 cases operated upon 1970-1997. Neurosurg Rev. 1998;21(2-3):126-137. doi:10.1007/BF02389318 PubMed | Crossref | Google Scholar
- Sayyahmelli S, Baran O, Uğurlar D, Kemerdere R, Antar V, Tanriverdi T. Intracranial intraventricular tumors: long-term surgical outcome of 25 patients. Turk J Med Sci. 2017;47(1):76-84. doi:10.3906/sag-1509-119
PubMed | Crossref | Google Scholar - Agarwal A, Kanekar S. Intraventricular Tumors. Semin Ultrasound CT MR. 2016;37(2):150-158. doi:10.1053/j.sult.2015.12.003 PubMed | Crossref | Google Scholar
- Brandes AA, Tosoni A, Franceschi E, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol. 2009;64(4):769-775. doi:10.1007/s00280-009-0926-8 PubMed | Crossref | Google Scholar
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. doi:10.1016/S1470-2045(09)70025-7 PubMed | Crossref | Google Scholar
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. 1949. The Clinical Evaluation of Chemotherapeutic Agents in Cancer
- Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704-710. doi:10.1093/jnci/85.9.704 PubMed | Crossref | Google Scholar
- Olson RA, Brastianos PK, Palma DA. Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J Neurooncol. 2011;105(2):325-335. doi:10.1007/s11060-011-0594-5 PubMed | Crossref | Google Scholar
Acknowledgments
Not reported
Funding
This study was conducted without any external funding.
Author Information
Corresponding Author:
Ashruta Patel
Department of Internal Medicine
Philadelphia College of Osteopathic Medicine, Georgia, USA
Email: ashrutapa@pcom.edu
Co-Author:
Robert Ayer
Department of Neurological Surgery
Apex Spine and Neurosurgery, Georgia, USA
Email: neurosurgery@robertayermd.com
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
The research ethical committee at Northside Hospital has waived institutional review board (IRB) approval. Informed consent was obtained and written from all participants.
Conflict of Interest Statement
The author declares no conflicts of interest or competing interests.
Guarantor
None
DOI
Cite this Article
Ashruta P, Robert A. Lateral Intraventricular Glioblastoma Presentation with Increased Survival Rate. medtigo J Neurol Psychiatry. 2025;2(1):e3084212. doi:10.63096/medtigo3084212 Crossref






