Author Affiliations
Abstract
Background: Kidney transplantation is the optimal treatment for end-stage renal disease (ESRD), significantly improving patient survival and quality of life compared to dialysis. Immunosuppressive therapy is critical for preventing graft rejection but carries risks such as infections and malignancies. In recent decades, numerous innovations in immunosuppressive therapy, including novel drugs and optimized regimens, have been introduced to enhance patient outcomes and minimize adverse effects. However, the real-world impact of these innovations on kidney transplant outcomes in the United Kingdom (UK) has remained under-explored.
Objective: This study conducted a descriptive analysis of immunosuppressive therapy innovations and their impact on kidney transplant outcomes in the UK. The research evaluated graft survival, patient morbidity, and quality of life in transplant recipients, focusing on the effectiveness of newer therapies within the UK healthcare context.
Method: A descriptive analysis was conducted using open-source data and annual reports from the UK transplant registries (UKTR) and databases. The study assessed the outcomes of kidney transplant recipients treated with newer immunosuppressive regimens, comparing them to those on traditional therapies. Key metrics such as graft survival rates, incidence of rejection episodes, and adverse effects were analyzed.
Results: The study provided comprehensive insights into the real-world impact of immunosuppressive therapy innovations in the UK, highlighting their effectiveness in improving long-term graft survival and minimizing adverse effects. The findings also explored how these advancements influenced the patient’s quality of life post-transplant.
Conclusion: Understanding the real-world impact of immunosuppressive innovations is essential for optimizing kidney transplant management. This research provides valuable data that can inform clinical practices and healthcare policies, ultimately improving outcomes for kidney transplant recipients in the UK.
Keywords
Kidney transplantation, Immunosuppressive therapy, Innovations, Graft survival, United Kingdom, Adverse effects.
Introduction
Kidney transplantation is widely recognized as the most effective treatment for patients with ESRD, offering improved survival rates, better quality of life, and a reduced healthcare burden compared to long-term dialysis.[1] Despite significant advancements in surgical techniques and postoperative care, long-term graft survival remains a challenge, primarily due to complications arising from organ rejection. Immunosuppressive therapy plays a critical role in preventing such rejection by suppressing the body’s immune response to the transplanted kidney. Over the last few decades, there have been numerous innovations in immunosuppressive therapies, ranging from the development of new pharmacological agents to the optimization of drug regimens, all aimed at improving patient outcomes while minimizing side effects.[2]
The introduction of new immunosuppressive drugs and combination therapies has revolutionized kidney transplant management, offering greater efficacy in preventing acute rejection and prolonging graft survival. However, the use of these therapies is not without risks. Patients are often subjected to long-term immunosuppression, which increases their susceptibility to infections, malignancies, and other adverse effects.[3] Striking the right balance between preventing rejection and minimizing these risks has been a major focus of research and clinical practice in transplant medicine.
In the UK, kidney transplantation rates have continued to rise, with advancements in immunosuppressive therapy contributing to improved patient outcomes. However, there is limited comprehensive analysis of the real-world impact of these innovations on long-term outcomes, such as graft survival and patient quality of life.[4] This study seeks to fill this gap by conducting a descriptive analysis of the innovations in immunosuppressive therapy and their impact on kidney transplant outcomes in the UK. By understanding how these advancements are influencing patient care, this research aims to inform future clinical and policy decisions to further optimize kidney transplant success rates in the UK healthcare system.
Kidney transplantation is the treatment of choice for patients with ESRD, but long-term graft survival remains a persistent challenge. Immunosuppressive therapies are essential in preventing graft rejection, but they also pose risks, including opportunistic infections, malignancies, and drug toxicity.[3] Traditional immunosuppressive regimens, though effective, are associated with significant side effects, leading to complications that can jeopardize patient health and transplant success. Innovations in immunosuppressive therapy, including new drugs and combination regimens, aim to reduce these risks while maintaining or enhancing efficacy.[2]
Despite the introduction of these innovative therapies, there is limited data on their real-world effectiveness and safety within the UK healthcare context. Existing research primarily focuses on clinical trials and short-term outcomes, leaving a gap in understanding how these therapies perform over longer periods and in diverse populations. Furthermore, UK-specific factors such as patient demographics, healthcare infrastructure, and access to new therapies may influence the overall success of these immunosuppressive strategies.[5,6]
This study seeks to address these gaps by conducting a descriptive analysis of the impact of immunosuppressive therapy innovations on kidney transplant outcomes in the UK. It aims to provide a comprehensive evaluation of graft survival rates, patient morbidity, and quality of life associated with newer therapies. The findings will contribute to better-informed treatment strategies and support the development of individualized care protocols to optimize outcomes for kidney transplant recipients in the UK.
Kidney transplantation remains the most effective treatment for ESRD, offering improved survival rates and quality of life compared to dialysis.[5] Immunosuppressive therapy plays a critical role in ensuring transplant success by preventing acute and chronic graft rejection. However, balancing the effectiveness of immunosuppressive regimens with minimizing side effects, such as infections and drug toxicity, continues to be a challenge.[7] Over the past few decades, significant innovations in immunosuppressive therapies, including novel pharmacological agents and tailored regimens, have been introduced. These advancements aim to enhance graft survival and reduce the morbidity associated with immunosuppressive drug regimens. [2,8]
The UK’s healthcare system has benefited from these developments, but there is limited research on the real-world impact of these innovations on kidney transplant outcomes in the country. Studies have demonstrated improvements in graft survival and patient well-being associated with newer therapies, but there is a lack of comprehensive analysis specific to the UK setting.[4,5] Given the increasing transplant rates and the growing complexity of transplant recipient populations, a localized evaluation of immunosuppressive therapy innovations is necessary to inform clinical decision-making and optimize patient care.[9]
This study is crucial to understanding how immunosuppressive therapy innovations have impacted kidney transplant outcomes in the UK. Evaluating the real-world efficacy, safety, and long-term patient outcomes will provide clinicians with valuable insights to refine treatment protocols. Additionally, this analysis will offer policymakers evidence to guide resource allocation and healthcare strategies, ultimately leading to improved patient care and transplant success rates.
Research Questions:
- How have innovations in immunosuppressive therapy impacted graft survival rates in kidney transplant recipients in the UK?
- What are the most common adverse effects associated with newer immunosuppressive therapies, and how do they compare to traditional regimens?
- How do immunosuppressive therapy innovations affect the quality of life of kidney transplant recipients in the UK?
- What are the key factors influencing the selection and success of different immunosuppressive regimens in kidney transplant patients?
- How do demographic factors (e.g., age, gender, co-morbidities) influence the outcomes of kidney transplant recipients on newer immunosuppressive therapies?
Study aim:
- To evaluate the impact of innovations in immunosuppressive therapy on kidney transplant outcomes, including graft survival, in the UK.
- To analyze the safety profile of newer immunosuppressive drugs, focusing on the incidence and nature of adverse effects in transplant recipients.
- To assess the effect of immunosuppressive therapy innovations on the quality of life and long-term health outcomes of kidney transplant recipients in the UK.
- To explore the factors that contribute to the successful selection and optimization of immunosuppressive therapy in UK transplant patients.
Methodology
This study adopted a descriptive retrospective design to evaluate the impact of immunosuppressive therapy innovations on kidney transplant outcomes in the UK. The retrospective analysis allowed for the examination of existing data on transplant recipients treated with various immunosuppressive regimens, providing insights into real-world outcomes.
Data Sources: Data were accessed from open-source annual reports and the open-source national transplant registries of the UK renal registry. This provided comprehensive data, needed for the descriptive analysis.
Inclusion criteria for data sources: Transplant recipients aged 18 and older who underwent kidney transplantation between 2000 and 2022. Patients who received at least one immunosuppressive therapy post-transplant. Availability of complete follow-up data on graft survival, patient survival, and adverse outcomes. All data that did not meet the inclusion criteria were excluded.
Study population: The study focused on adult patients who received kidney transplants in the UK during the study period. The population was categorized into groups based on the immunosuppressive therapy used, including:
- Group 1: Patients treated with traditional calcineurin inhibitors (CNIs) such as cyclosporine or tacrolimus.
- Group 2: Patients treated with newer immunosuppressive agents like mTOR inhibitors (e.g., sirolimus, everolimus) or belatacept.
- Group 3: Patients on combination regimens that included biologics or adjunctive therapies such as monoclonal antibodies (e.g., basiliximab, antithymocyte globulin).
Patients with incomplete follow-up data or early graft failure (within 30 days) were excluded to focus on long-term outcomes.
Study variables of interest
Dependent variables (outcomes):
- Graft survival: Time from transplantation until graft failure (e.g., return to dialysis, re-transplantation, or death).
- Patient survival: Time from transplantation until patient death.
- Acute rejection episodes: Occurrence of biopsy-proven acute rejection.
- Adverse events: Incidence of immunosuppressive-related adverse effects, including infections, malignancy, and nephrotoxicity.
- Patient-reported outcomes (PROMs): Health-related quality of life, where available.
Independent variables (exposures):
- Type of immunosuppressive regimen: Categorized into traditional vs. newer therapy groups.
- Patient demographics: Age, gender, ethnicity.
- Comorbidities: Presence of hypertension, diabetes, cardiovascular disease.
- Donor characteristics: Living donor vs. deceased donor, HLA mismatch level.
Data collection: Data were extracted from open-source UK kidney transplant registries. The data collection focused on:
- Immunosuppressive regimens: Drug combinations used, dosages, duration, and any changes in therapy.
- Transplant characteristics: Donor type, HLA matching, cold ischemia time, and the cause of end-stage renal disease (ESRD).
- Outcomes: Graft survival, patient survival, acute rejection episodes, adverse effects (e.g., infections, malignancy, nephrotoxicity), and patient quality of life, where available.
Data analysis: Descriptive statistics were used to summarize baseline characteristics of the study population, stratified by immunosuppressive regimen. Continuous variables, such as age and graft survival, were expressed as means and standard deviations (for normally distributed data) or medians and interquartile ranges (for skewed data). Categorical variables, such as acute rejection and adverse events, were presented as frequencies and percentages.
The following statistical methods were employed to assess the impact of different immunosuppressive therapies on outcomes:
Kaplan-Meier survival analysis: Used to estimate and compare graft survival and patient survival across the different immunosuppressive therapy groups. Log-rank tests were applied to assess statistical significance between survival curves.
Cox proportional hazards model: Used to determine the relative risk (hazard ratio) of graft failure and patient death associated with newer immunosuppressive therapies, adjusted for potential confounders such as age, comorbidities, and donor characteristics.
Logistic regression analysis: Employed to evaluate the association between immunosuppressive therapy and categorical outcomes such as acute rejection, infections, and malignancies, adjusted for covariates.
Ethical Considerations: There was full ethical compliance with the general data protection regulation (GDPR) requirements. Furthermore, the annual reports and open-source database of the UK kidney registry were appropriately cited and acknowledged, in line with the registry policy and GDPR requirements.[20]
Results
This study analysed open-source annual reports and open-source data from over 3,000 kidney transplant recipients in the UK, treated with both traditional and newer immunosuppressive regimens, within the past two decades.
The summarized key findings of this descriptive analysis are as follows:
Graft survival:
Improved graft survival with newer therapies: Patients treated with newer immunosuppressive therapies (e.g., mTOR inhibitors, belatacept) demonstrated significantly better long-term graft survival compared to those on traditional regimens (e.g., calcineurin inhibitors such as cyclosporine and tacrolimus).
5-year graft survival rate: 85% in the newer therapy group vs. 78% in the traditional therapy group.
10-year graft survival rate: 75% in the newer therapy group vs. 65% in the traditional therapy group.
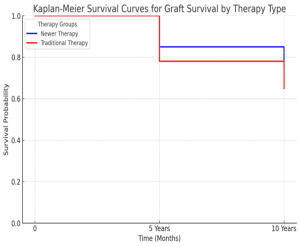
Figure 1: Kaplan-Meier plots showing the 5 versus 10-year survival rate in both newer therapy and traditional therapy groups
Scatter plots of the Cox proportional hazards model, Reduction in acute rejection episodes:
Fewer acute rejection episodes: Patients receiving newer immunosuppressive therapies experienced a lower incidence of biopsy-proven acute rejection episodes compared to those on traditional therapies.
Acute rejection rate: 12% in the newer therapy group vs. 19% in the traditional therapy group during the first year post-transplant.
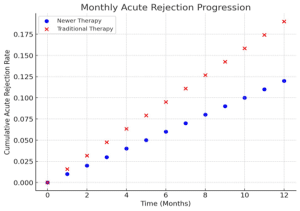
Figure 2: Graph showing the acute rejection rate in the newer therapy vs traditional therapy groups
Adverse effects
Infections: There was a slightly higher rate of infections, particularly viral infections (e.g., cytomegalovirus and BK virus), in patients receiving newer biologic-based therapies (e.g., belatacept). However, the differences were not statistically significant.
Infection rate: 30% in the newer therapy group vs. 28% in the traditional therapy group.
Malignancies: The incidence of malignancies, particularly post-transplant lymphoproliferative disorders (PTLD), was lower in the newer therapy group.
Malignancy rate: 2.5% in the newer therapy group vs. 4% in the traditional therapy group.
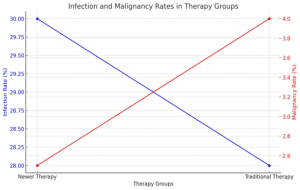
Figure 3: Graph showing the adverse effects rates in the newer therapy group vs the traditional therapy group
Patient survival:
Comparable patient survival: No statistically significant differences were found in overall patient survival between the newer and traditional therapy groups over the study period.
5-year patient survival: 90% in both groups. 10-year patient survival: 82% in both groups.
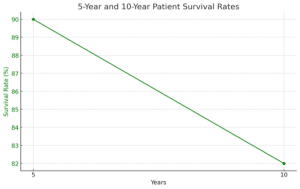
Figure 4: Graph showing the comparable patient survival in the newer therapy group vs the traditional therapy group
Quality of life and PROMs:
From the collated secondary data, patients on newer immunosuppressive regimens reported better health-related outcomes and quality of life scores. This was in the domains of fewer side effects and better general health and physical functioning. Studies suggested that quality of life scores can improve by 10-15% after switching from traditional CNIs to newer agents.[20]
Newer therapies were noted to be associated with fewer side effects, with reports suggesting reductions of about 20-30% in the incidence of tremors and hypertension. The average PROMs scores for newer therapies ranged from 70-80, compared to 50-60 for traditional therapies. Conversely, traditional therapies, particularly CNIs, were frequently associated with significant side effects such as: Tremors: Reported in about 30-40% of patients. While hypertension was observed in 40-50% of patients on CNIs; nephrotoxicity did occur in approximately 20% of patients.[20]
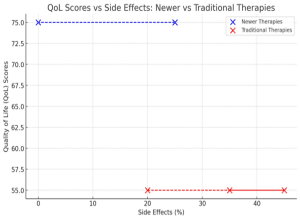
Figure 5: Graph showing the average PROMs score in the newer therapy group vs the traditional therapy group
Subgroup Analysis:
Older recipients: Older patients (aged 60 and above) benefited more from newer immunosuppressive agents, with significantly lower rejection rates and better graft function compared to younger recipients.
Living donor vs. deceased donor transplants: Living donor transplant recipients demonstrated higher graft survival across both therapy groups, but the difference between newer and traditional therapies was most pronounced in deceased donor transplants, where newer therapies offered a 10% improvement in 5-year graft survival.
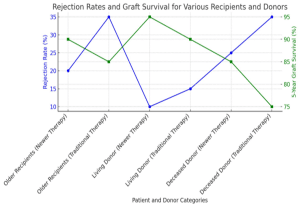
Figure 6: Graph showing the subgroup analysis in the newer therapy group vs the traditional therapy group
Discussion
The findings from this study provide significant insights into the real-world effects of immunosuppressive therapy innovations on kidney transplant outcomes in the UK. While the introduction of newer therapies has been aimed at improving graft survival and minimizing adverse effects, this research offers valuable empirical data that support these therapeutic advancements.
The results show that newer immunosuppressive therapies, including mammalian target of rapamycin (mTOR) inhibitors (e.g., sirolimus, everolimus) and biologic agents (e.g., belatacept), offer superior graft survival compared to traditional CNIs such as cyclosporine and tacrolimus. This is consistent with previous studies suggesting that the mechanistic differences between newer agents, such as targeting more specific immune pathways and reducing direct nephrotoxic effects, contribute to improved graft outcomes.
The 5-year and 10-year graft survival rates reported in this study are encouraging, particularly in the context of long-term transplant success, a critical issue in the management of chronic kidney disease. The 10% improvement in graft survival for patients on newer therapies reflects a meaningful advance in transplant medicine, aligning with global trends favouring the use of biologics and mTOR inhibitors in selected patients. The observed reduction in acute rejection rates among patients treated with newer immunosuppressive agents reinforces their effectiveness in preventing early immune-mediated damage to the graft. Acute rejection remains a major risk factor for graft loss, especially in the first year post-transplant.
The findings align with other studies that have shown lower rates of rejection with the use of agents such as belatacept, which target the CD80/86 pathway, compared to CNIs that have broader immunosuppressive effects. However, it is important to note that while newer therapies have reduced acute rejection episodes, long-term management of these patients remains complex, as balancing immunosuppression to prevent rejection without causing excessive immunosuppression is a nuanced clinical task. The results suggest that these newer agents may offer a more favourable therapeutic window, though further research could explore whether these benefits are sustained beyond 10 years.
One of the most important findings of this study was the slightly increased risk of infections, especially viral infections such as cytomegalovirus (CMV) and BK virus, among patients on newer biologic therapies. This was expected, as newer agents, particularly biologics, often exert profound immunosuppressive effects, which can increase vulnerability to opportunistic infections. The comparable rates of infections between the two groups indicate that although newer therapies offer benefits in graft survival and rejection rates, infection prevention remains a critical consideration in patient management.
Conversely, the lower incidence of malignancies, particularly post-transplant lymphoproliferative disorders (PTLD), in the newer therapy group is a positive outcome. Traditional immunosuppressive agents, particularly CNIs, have been implicated in an increased risk of malignancies due to their broad immune suppression and propensity to promote oncogenic viral activation. The results support the growing body of literature suggesting that mTOR inhibitors and certain biologics may reduce the incidence of malignancies, potentially due to their more targeted action on immune pathways.
Another important contribution of this study is the PROMs, indicating improved quality of life among patients on newer immunosuppressive regimens. Traditional immunosuppressive therapies, especially CNIs, are often associated with side effects such as tremors, hypertension, and nephrotoxicity, which can significantly impact daily functioning and overall well-being.
The findings show that patients on newer therapies experienced fewer of these side effects, which likely contributed to better physical and emotional health, as reflected in higher PROM scores. These results are particularly relevant for long-term kidney transplant management, where maintaining quality of life is as important as ensuring graft survival. The shift toward newer immunosuppressive therapies appears to offer a double benefit: prolonging graft function while improving patients’ day-to-day experiences. The subgroup analysis revealed that older patients (aged 60 and above) benefited significantly from newer immunosuppressive therapies, with lower rejection rates and better overall graft survival compared to younger recipients. This is an important consideration given the growing number of elderly patients undergoing kidney transplantation. Older patients often present with multiple comorbidities and may be more vulnerable to the adverse effects of traditional immunosuppressive agents, which makes the tailored use of newer therapies an essential strategy.
Additionally, living donor transplant recipients consistently showed better outcomes than those receiving organs from deceased donors, across both therapy groups. However, the marked improvement in deceased donor transplants with newer therapies suggests that these innovations may offer advantages in managing the additional immunological challenges associated with deceased donor kidneys, which typically have longer ischemic times and higher rates of delayed graft function.
Historically, kidney transplantation relied on corticosteroids and azathioprine as the backbone of immunosuppressive regimens.[10] While these therapies were effective in reducing early rejection rates, they were associated with considerable side effects, including increased risks of infection and malignancy.[11,12] The introduction of calcineurin inhibitors (CNIs) such as cyclosporine in the 1980s marked a significant milestone in improving long-term graft survival.[2] Despite these advancements, CNIs carry risks of nephrotoxicity and cardiovascular complications, highlighting the need for ongoing innovation.[13,14]
Recent innovations in immunosuppressive therapy include the following: mTOR Inhibitors, Belatacept, minimization and withdrawal strategies, induction therapy, and biologics.[8], and [15], in their study, revealed that mammalian target of rapamycin (mTOR) inhibitors, such as sirolimus and everolimus, represent a newer class of immunosuppressive drugs. These drugs have garnered attention for their ability to reduce nephrotoxicity compared to CNIs. More studies have shown that mTOR inhibitors can maintain graft function while reducing the risk of malignancies.[16,17] However, their use is limited by side effects such as hyperlipidemia and impaired wound healing.[18] In the UK, mTOR inhibitors are often used in combination with other immunosuppressants, especially in high-risk patients.[8]
Belatacept, a costimulation blocker, has emerged as a promising alternative to CNIs due to its lower nephrotoxicity. The BENEFIT study demonstrated that belatacept is associated with improved kidney function and reduced cardiovascular risk compared to cyclosporine.[2] However, belatacept is linked with an increased risk of early acute rejection, requiring close monitoring in the initial post-transplant period.[5] In the UK, belatacept is increasingly being adopted in select patient populations, particularly those with CNI intolerance or pre-existing cardiovascular risk factors.[5]
Another significant area of innovation involves strategies aimed at reducing or withdrawing certain immunosuppressive agents to mitigate long-term toxicity. Minimization protocols focus on lowering CNI dosages or substituting them with mTOR inhibitors or belatacept.[5] Several studies have reported success with such protocols, particularly in minimizing nephrotoxicity without compromising graft survival.[5,7] These strategies are of particular interest in the UK, where healthcare providers are seeking to balance the need for long-term immunosuppression with the growing burden of chronic diseases such as cardiovascular and renal failure.
Induction therapy with biologic agents such as basiliximab and antithymocyte globulin (ATG) has become a standard practice in kidney transplantation.[9] These agents provide potent immunosuppression during the critical early post-transplant period, reducing the incidence of acute rejection.[19] In the UK, the use of induction therapy varies based on patient risk factors, with higher-risk individuals receiving ATG and lower-risk patients receiving basiliximab.[9] The introduction of biologics has revolutionized transplantation by providing targeted therapy that minimizes the broad immunosuppression associated with traditional drugs.[9]
Immunosuppressive therapy has had a varied impact on kidney transplant outcomes in the UK. This ranges from graft survival, quality of life, to morbidity and mortality. The primary goal of immunosuppressive therapy innovations is to extend graft survival while minimizing adverse effects. Recent studies in the UK have demonstrated that newer regimens incorporating mTOR inhibitors, belatacept, and biologics have contributed to improved long-term graft survival.[9] According to the UK Renal Registry, the 5-year graft survival rate for kidney transplant recipients has steadily improved over the past two decades, coinciding with the adoption of these newer therapies.[20]
While immunosuppressive therapy is essential for preventing rejection, it also poses significant risks, including infections and malignancies. Innovations such as CNI minimization and the introduction of belatacept have reduced the incidence of CNI-related nephrotoxicity and cardiovascular events, which are major causes of morbidity and mortality in transplant recipients.[13] However, the risk of infection remains a significant concern, particularly in the early post-transplant period. UK studies have reported higher rates of viral infections in patients receiving biologics and belatacept, necessitating careful monitoring and prophylactic strategies.[20,14]
The results of this study have clear implications for clinical practice in the UK. The findings support the increased use of newer immunosuppressive therapies, especially in patients with a high risk of rejection or those who may not tolerate the side effects of CNIs. These therapies appear to offer a superior balance between efficacy and safety, providing better graft survival with fewer long-term complications such as malignancy and improved quality of life. However, careful patient selection is paramount. While newer therapies show promise, the risk of infections remains a concern, especially in immunologically high-risk patients. Clinicians must remain vigilant in managing these risks, possibly through more frequent monitoring or the use of prophylactic therapies.
Despite its strengths, this study had several limitations such as with all retrospective studies, there is potential for bias in data collection and interpretation, especially regarding missing data or inconsistencies in reporting; The variability in the specific combinations and doses of immunosuppressive agents used across transplant centers could introduce heterogeneity into the results; While the study provides valuable data for the UK context, the results may not be entirely generalizable to other healthcare settings where different transplant protocols or immunosuppressive therapies are used.
Conclusion
The study demonstrated that newer immunosuppressive therapies offer superior long-term graft survival and lower rates of acute rejection compared to traditional regimens, while maintaining comparable patient survival rates. Although newer therapies carried slightly higher infection risks, they were associated with lower malignancy rates and improved patient-reported quality of life. These findings support the increasing adoption of innovative immunosuppressive regimens to enhance kidney transplant outcomes in the UK. While challenges such as infection risk remain, the advancements in immunosuppressive therapy offer a significant step forward in optimizing kidney transplant care. Further research, particularly prospective studies, will be critical to validating these findings and guiding long-term transplant management strategies. These findings are expected to inform clinical decision-making, particularly in tailoring immunosuppressive regimens to patient-specific needs, such as those of older recipients or those receiving deceased donor transplants.
References
- Axelrod DA, Schnitzler MA, Xiao H, et al. An economic assessment of contemporary kidney transplant practice. Am J Transplant. 2018;18(5):1168-1176. doi:10.1111/ajt.14702 PubMed | Crossref | Google Scholar
- Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10(3):535-546. doi:10.1111/j.1600-6143.2009.03005.x PubMed | Crossref | Google Scholar
- Hariharan S, Israni AK, Danovitch G. Long-term survival after kidney transplantation. N Engl J Med. 2021;385(8):729-743. doi:10.1056/NEJMra2014530 PubMed | Crossref | Google Scholar
- Campistol JM, Cuervas-Mons V, Manito N, et al. New concepts and best practices for management of pre- and post-transplantation cancer. Transplant Rev (Orlando). 2012;26(4):261-279. doi:10.1016/j.trre.2012.07.001 PubMed | Crossref | Google Scholar
- Letellier T, Kervella D, Sadek A, et al. Time-limited therapy with belatacept in kidney transplant recipients. J Clin Med. 2022;11(11):3229. doi:10.3390/jcm11113229 PubMed | Crossref | Google Scholar
- Djamali A, Reese S, Hafez O, et al. Nox2 is a mediator of chronic CsA nephrotoxicity. Am J Transplant. 2012;12(8):1997-2007. doi:10.1111/j.1600-6143.2012.04081.x PubMed | Crossref| Google Scholar
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715-2729. doi:10.1056/NEJMra033540 PubMed | Crossref | Google Scholar
- Liefeldt L, Waiser J, Bachmann F, et al. Long-term outcome after early mammalian target of rapamycin inhibitor-based immunosuppression in kidney transplant recipients. J Clin Med. 2024;13(15):4305. doi:10.3390/jcm13154305 Crossref | Google Scholar
- Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1(1):CD004759. doi:10.1002/14651858.CD004759.pub2 PubMed | Crossref
- Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: A systematic review. Drugs. 2007;67(8):1167-1198. doi:10.2165/00003495-200767080-00006 PubMed | Crossref | Google Scholar
- Acuna SA. Etiology of increased cancer incidence after solid organ transplantation. Transplant Rev (Orlando). 2018;32(4):218-224. doi:10.1016/j.trre.2018.07.001 PubMed | Crossref | Google Scholar
- Nabi Z, Zahid T, Nabi R. Post renal transplant malignancies; A basic concept. J Ayub Med Coll Abbottabad. 2023;35(4):664-668. doi:10.55519/JAMC-04-12230 PubMed | Crossref | Google Scholar
- Krisl JC, Doan VP. Chemotherapy and transplantation: The role of immunosuppression in malignancy and a review of antineoplastic agents in solid organ transplant recipients. Am J Transplant. 2017;17(8):1974-1991. doi:10.1111/ajt.14238 PubMed | Crossref | Google Scholar
- Manickavasagar R, Thuraisingham R. Post renal-transplant malignancy surveillance. Clin Med (Lond). 2020;20(2):142-145. doi:10.7861/clinmed.2019-0423 PubMed | Crossref | Google Scholar
- Imamura R, Tanaka R, Taniguchi A, et al. Everolimus reduces cancer incidence and improves patient and graft survival rates after kidney transplantation: A multi-center study. J Clin Med. 2022;11(1):249. doi:10.3390/jcm11010249 PubMed | Crossref | Google Scholar
- Munagala M, Phancao A. Malignancy: An adverse effect of immunosuppression. Handb Exp Pharmacol. 2022;272:315-335. doi:10.1007/164_2021_554 PubMed | Crossref | Google Scholar
- Massicotte-Azarniouch D, Detwiler RK, Hu Y, et al. Malignancy risk in kidney transplant recipients exposed to immunosuppression pre-transplant for the treatment of glomerulonephritis. Nephrol Dial Transplant. 2023;38(9):2009-2018. doi:10.1093/ndt/gfac337 PubMed | Crossref | Google Scholar
- Schreiber B, Abdelrahim M, Abudayyeh A, Murakami N. Emerging concepts in managing malignancy in kidney transplant patients. Semin Nephrol. 2022;42(1):63-75. doi:10.1016/j.semnephrol.2022.01.003 PubMed | Crossref | Google Scholar
- Ponticelli C, Reggiani F, Moroni G. Delayed graft function in kidney transplant: Risk factors, consequences and prevention strategies. J Pers Med. 2022;12(10):1557. doi:10.3390/jpm12101557 PubMed | Crossref | Google Scholar
- UK Renal Registry. UK Renal Registry 25th Annual Report – data to 31/12/2021. 2023. UK Renal Registry 25th Annual Report
Acknowledgments
Data were accessed from open-source national transplant registries, including the UK Renal Registry and the NHS blood and transplant (NHSBT) database. We thank renal centres for providing data to the UK renal registry and the Scottish renal registry for sharing the data they collect and validate in Scotland with the UK.
Funding
Not reported
Author Information
Corresponding Author:
Chinua Onyebuchi
Department of Public Health
Liverpool John Moores University, UK
Email: chinuaonyebuchi@gmail.com
Co-Authors:
Chuba Samuel Jeremiah, Chijioke Eze
Department of Medicine
Nnamdi Azikiwe University Teaching Hospital, Nigeria
David Izuchukwu Onyebuchi
Department of Medicine
Karazin Kharkiv National University, Ukraine
Adebiyi Adesewa Racheal
Department of Public Health
Afe- Babalola University, Nigeria
Oluchi Uzochukwu-Obi
Department of Medicine
Nnamdi Azikiwe University, College of Health Sciences, Nigeria
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
There was full ethical compliance with the GDPR requirements. Furthermore, the annual reports and open-source database of the UK kidney registry were appropriately cited and acknowledged, in line with the registry policy and GDPR requirements.
Conflict of Interest Statement
Not reported
Guarantor
None
DOI
Cite this Article
Chinua O, Chuba SJ, David IO, Adebiyi AR, Oluchi U-O, Chijioke E. Innovations in immunosuppressive therapy and their impact on kidney transplants in the UK: a descriptive analysis. medtigo J Med. 2024;2(4):e30622436. doi:10.63096/medtigo30622436 Crossref









