Author Affiliations
Abstract
Introduction: The determination of physical compatibility of intravenous (IV) medications is crucial when multiple agents are co-administered to ensure patient safety and therapeutic effectiveness. The physical compatibility of doxycycline hyclate and acyclovir sodium has not been well studied at various concentrations, which currently limits their potential for safe co-administration.
Objective: To determine the in vitro physical compatibility of doxycycline hyclate 10 mg/mL and acyclovir 5 mg/mL.
Methodology: The admixtures were prepared with 0.9% sodium chloride (NaCl), mixing equal volumes of doxycycline hyclate (10 mg/mL) and acyclovir sodium (5 mg/mL). Admixtures were examined at baseline, 1, 5, 8, and 24 hours. Physical incompatibility was defined as a demonstrable change in absorbance (> 0.010 units), pH (> 0.1 unit), or in visual appearance.
Results: All admixtures exhibited a medium haze at baseline and the first hour. At hour 5, the admixture had formed a precipitate which persisted until the end of the 24-hour study. The absorbance for each admixture revealed a demonstrable change by the first hour. This demonstrable change in absorbance continued throughout the study.
Conclusion: This study found that doxycycline hyclate and acyclovir sodium are not physically compatible at the concentrations tested. Due to the formation of a strong visual precipitate upon mixing, we strongly recommend that this admixture not be administered to patients, as it may compromise safety.
Keywords
Doxycycline hyclate, Acyclovir sodium, Admixtures, Physical compatibility, Sodium chloride.
Introduction
The physical compatibility of IV drugs and fluids is crucial for the safe and effective administration of IV therapies to hospitalized patients.[1] Incompatibilities between co-administered IV medications can lead to precipitation, turbidity, color change, or potency loss, which may result in catheter occlusion or severe harm to the patient.[2,3] Drug stability and physical compatibility are especially critical in patients requiring multiple simultaneous infusions. This becomes particularly relevant when considering specific medications such as doxycycline hyclate and acyclovir that may require concomitant administration.
Doxycycline hyclate is a tetracycline antibiotic that can be prescribed to treat respiratory infections, rickettsia, sexually transmitted diseases, and for malaria prophylaxis.[4] Acyclovir is an antiviral medication indicated to treat viral infections, including chickenpox, shingles, and herpes.[5] The physical compatibility of IV doxycycline hyclate (1 mg/ml) and acyclovir (5 mg/ml) was assessed by Forman et al. in 5% dextrose water (D5W) for four hours.[6] The study found this admixture in D5W to be physically compatible for up to 4 hours. Despite their common use in hospitalized patients, limited information exists regarding the compatibility of doxycycline hyclate and acyclovir during concomitant IV administration at other concentrations. Therefore, this study aimed to determine the in vitro physical compatibility of the IV formulations of doxycycline hyclate 10 mg/mL and acyclovir 5 mg/mL, assessing visual appearance, pH, and potential precipitation over time.
Methodology
The methods used in this in vitro study, which simulates Y-site administration, have been previously published.[7] Using an aseptic technique, doxycycline hyclate was reconstituted to 10 mg/mL per the manufacturer’s recommendations for the commonly used dosage. To prepare the admixture, 70 mL of the reconstituted doxycycline hyclate was withdrawn and transferred to an empty NS 250 mL IV bag (Fresenius Kabi, Lake Zurich, IL) using a 5-micron filter needle (Becton Dickenson and Company, Franklin Lakes, NJ). Acyclovir was diluted according to the manufacturer’s directions to 5 mg/mL for the commonly used dosage. The same procedure was used to transfer 70 mL of acyclovir to the admixture IV bag, resulting in a 1:1 ratio of doxycycline and acyclovir.
After gently mixing the admixture, 3 mL aliquots were placed into polystyrene cuvettes (Fisherbrand, Pittsburgh, PA) and covered with parafilm. Additionally, 5 mL aliquots of the admixture were placed into capped polystyrene test tubes (Fisherbrand, Pittsburgh, PA). All aliquots were prepared in triplicate at room temperature under ambient light for each time point (baseline, 1, 5, 8, and 24 hours).
To assess the admixture absorbance, we examined the aliquots using a Genesys 10S UV-Visible spectrophotometer (Thermo Fisher Scientific, Waltham, MA). A 0.9% NaCl sample was used as the control, with a setting of 547 nm, which corresponds to the wavelength of 100% transmittance for 0.9% NaCl and the drugs. A change in absorbance of >0.01 units from baseline was considered a demonstrable change. The admixture pH was examined using a 7-compact pH meter with an InLab science pH electrode (Mettler Toledo, Columbus, OH). A demonstrable change in pH was a change >0.1 from baseline.
Visual changes were examined against a white and black background using a scale from 0-4: no precipitate (0), trace evidence of precipitation (1), slight haze (2), medium haze (3), and heavy precipitate (4). The positive control was prepared with equal volumes of calcium gluconate and sodium phosphate. Any change in color was also evaluated and recorded by the original investigator and confirmed by a second investigator as needed. The temperature and humidity of the room were recorded at each time point.
Results
All admixture results can be found in Table 1 and Figure 1. Spectrophotometric analysis of admixtures revealed an absorbance change ≥ 0.01 from baseline to all time points throughout the 24-hour study period. The pH of the admixtures remained stable during the investigation with no pH excursions ≥ 0.1 units. At hour 0, just after mixing, the admixture became hazy and remained hazy at hour 1. A precipitate was visible in the admixtures at hours 5, 8, and 24. When the admixture was examined against a black background, a precipitate was noticeably prevalent. At hour 24, the admixture showed increased cloudiness with a yellow tint. During the investigation, the temperature range was 22.1 – 22.5 °C and the humidity was 64 – 65%.
| Variable and sample of Doxycycline* & Acyclovir sodium ‡ admixture |
Study time (hr) ____________________________________________________ 0 1 5 8 24 |
||||
| Absorbance units | |||||
| A | 0.323 | 1.840 | 2.007 | 2.146 | 2.130 |
| B | 0.410 | 1.848 | 2.008 | 2.161 | 2.375 |
| C | 0.500 | 1.838 | 2.065 | 2.118 | 2.397 |
| Average ± SD | 0.41 ± 0.089 | 1.84 ± 0.005 | 2.03 ± 0.033 | 2.14 ± 0.022 | 2.30 ± 0.148 |
| pH | |||||
| A | 4.43 | 4.46 | 4.46 | 4.46 | 4.52 |
| B | 4.37 | 4.40 | 4.40 | 4.35 | 4.45 |
| C | 4.36 | 4.39 | 4.39 | 4.32 | 4.45 |
| Average ± SD | 4.39 ± 0.04 | 4.42 ± 0.04 | 4.42 ± 0.04 | 4.38 ± 0.07 | 4.47 ± 0.04 |
| Visual scale | |||||
| A | 3 | 3 | 4 | 4 | 4 |
| B | 3 | 3 | 4 | 4 | 4 |
| C | 3 | 3 | 4 | 4 | 4 |
| Average ± SD | 3 ± 0 | 3 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 |
Table 1: Absorbance, pH, and visual admixture results
* Doxycycline 10mg/mL, Mylan, Rockford, IL. Lot number 7008897
‡ Acyclovir Sodium 5mg/mL, Fresenius Kabi, Lake Zurich, IL. Lot number 6023933
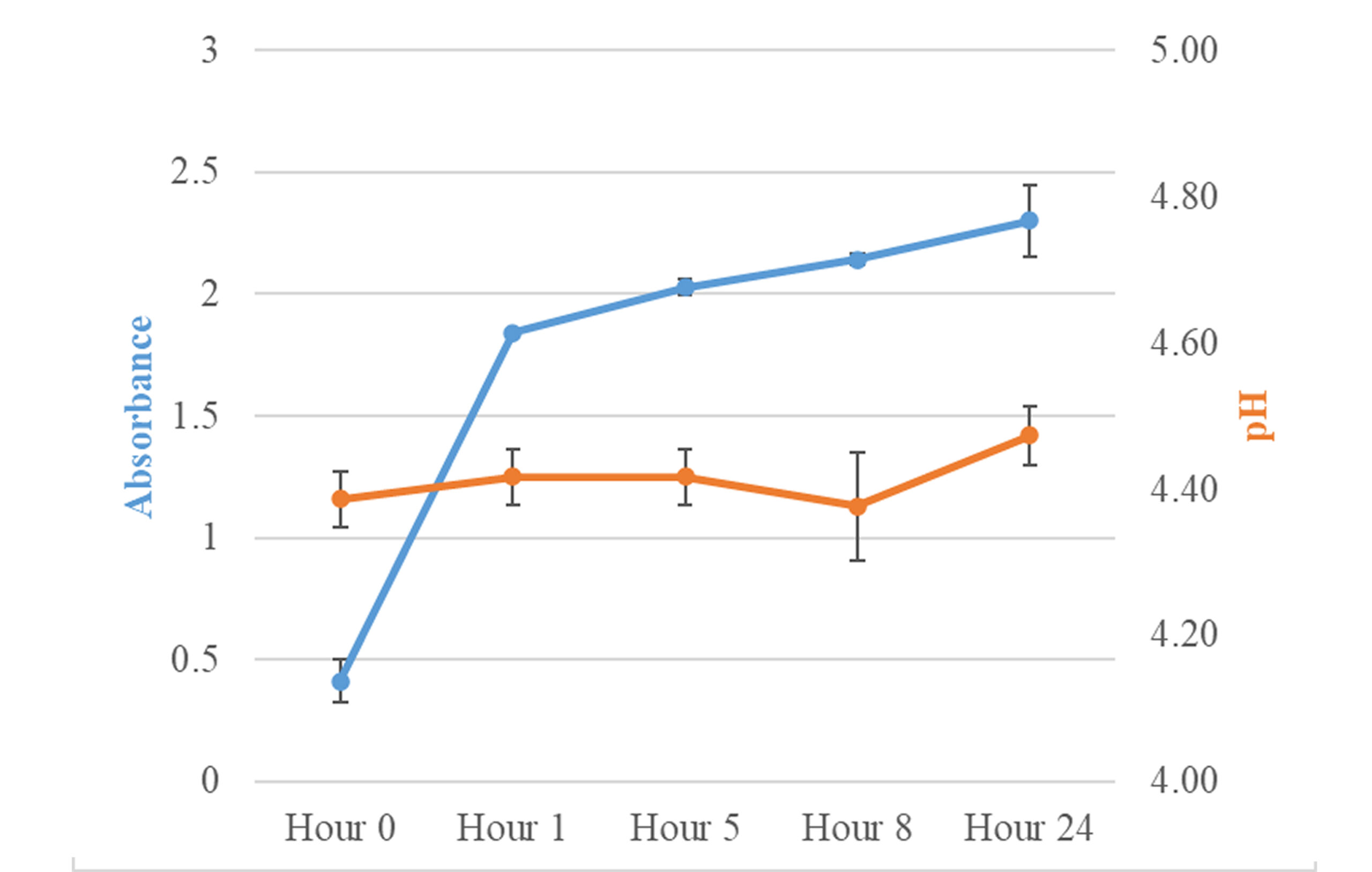
Figure 1: Summary of acyclovir – doxycycline compatibility results (values are displayed as average and standard deviation)
Discussion
This in vitro study builds on earlier IV compatibility information of the same drugs in a lower concentration (5 mg/ml of acyclovir and 1 mg/ml of doxycycline hyclate) in D5W conducted by Forman and colleagues.[6] In that earlier investigation, the drugs were deemed physically compatible in D5W for up to 4 hours. Our study examined a higher concentration of doxycycline, over a longer period, to better simulate the duration over which IV admixtures might be co-administered in a hospital setting.
There was evidence of haze at baseline, and precipitation was noted at hour 5, accompanied by an increase in absorbance throughout the study duration. These findings indicate physical incompatibility between the drugs at the studied concentrations.
Although we planned our in vitro study to mimic bedside clinical administration, several limitations must be acknowledged. First, chemical stability was not assessed using high-performance liquid chromatography. Therefore, conclusions are limited to physical compatibility observations and do not consider the potential degradation or loss of potency of either drug over the study period. Second, the absence of a Y-site flow simulation in the study design limits extrapolation to real-world co-administration conditions, where dynamic flow rates and dilution effects could alter drug interactions. Further investigation may be warranted using additional analytical methods to quantitatively assess the chemical stability and compatibility of the drugs to confirm the extent of the incompatibility identified in this study.
Conclusion
Doxycycline hyclate and acyclovir had demonstrable changes. Based on our findings, the admixture of doxycycline 10 mg/mL and acyclovir 5 mg/mL is not physically compatible, and we recommend that it should not be administered concomitantly to patients.
References
- Wolters Kluwer. Lexicomp. Lexicomp
- Newton DW. Y-site Compatibility of Intravenous Drugs with Parenteral Nutrition. JPEN J Parenter Enteral Nutr. 2013;37(3):297-299. doi:10.1177/0148607112465653 PubMed | Crossref | Google Scholar
- Allen LV, Popovich NG, Ansel HC. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. 12th ed. Lippincott Williams & Wilkins, a Wolters Kluwer business; 2023. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems
- VIBRAMYCIN® doxycycline hyclate for injection. Reference ID: 3371402. 2013. VIBRAMYCIN® doxycycline hyclate for injection
- Fresenius Kabi USA. Acyclovir sodium injection. Acyclovir sodium injection
- Forman JK, Lachs JR, Souney PF. Visual compatibility of acyclovir sodium with commonly used intravenous drugs simulated Y-site injection. Am J Hosp Pharm. 1987;44(6):1408-1409. Visual compatibility of acyclovir sodium with commonly used intravenous drugs simulated Y-site injection
- Maktabi B, Howard MS, Baki G, Churchwell MD. Intravenous Physical Compatibility of Heparin Sodium and Furosemide. Int J Pharm Compd. 2022;26(6):522-526. Intravenous Physical Compatibility of Heparin Sodium and Furosemide
Acknowledgments
The authors would like to acknowledge the support of the University of Toledo College of Pharmacy & Pharmaceutical Sciences for their valuable contributions to this study.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Information
Corresponding Author:
Mitchell S Howard
Department of Pharmacy Practice
University of Toledo College of Pharmacy and Pharmaceutical Sciences, USA
Email: mitchell.howard@utoledo.edu
Co-Authors:
Kylie B Grant, Mariann D Churchwell, Gabriella Baki
Department of Pharmacy Practice
University of Toledo College of Pharmacy and Pharmaceutical Sciences, USA
Authors Contributions
All authors contributed equally to the research design, methodology, data curation, analysis, and preparation of the manuscript (original and revisions).
Ethical Approval
This article does not contain any studies with human or animal participants.
Conflict of Interest Statement
The authors declared no conflicts of interest.
Guarantor
The guarantor of this work is Mitchell S. Howard, USA.
DOI
Cite this Article
Grant KB, Churchwell MD, Baki G, Howard MS. In-Vitro Physical Compatibility of Intravenous Doxycycline Hyclate and Acyclovir Sodium. medtigo J Pharmacol. 2025;2(4):e3061242. doi:10.63096/medtigo3061242 Crossref









