Author Affiliations
Abstract
Moringa oleifera, also known as the miracle tree, is considered nutritionally rich with therapeutic properties. This article emphasizes the phytochemicals and their therapeutic potential in the management of Type II diabetes. This study aims to provide a comprehensive understanding of Moringa oleifera’s contributions to the management of diabetes. Nowadays, attention has been drawn towards Moringa for its potential to manage oxidative stress and inflammation associated with Type II diabetes. In this review article, we included various scientific studies to establish a link between the effect of moringa extracts on metabolic parameters and Type II diabetes. The studies indicated that Moringa extracts improve glucose tolerance and insulin signaling. It is seen that the moringa extracts can reduce plasma insulin levels and pro-inflammatory cytokines. Experimental models such as high-fat diet-fed and streptozocin-induced diabetic animals have shown decreased fasting glucose levels and improved lipid profiles on the administration of Moringa extracts. In addition, it has demonstrated improvements in body weight and a decrease in inflammatory markers in obese and diabetic animal models. Furthermore, the extract has shown effectiveness in wound healing and improved insulin sensitivity, which indicates its multifaceted use in diabetes management. In a nutshell, the findings of scientific research indicate that Moringa extracts can be used for managing Type II diabetes. However, there is a need for more extensive research to fully understand and elucidate the mechanism of action and clinical implications of Moringa oleifera in diabetes.
Keywords
Moringa oleifera, Type II diabetes, Oxidative stress, Inflammation, Antioxidants, Blood glucose, Preclinical studies.
Introduction
Moringa oleifera belongs to the Moringaceae family and has been used for its nutritional and therapeutic benefits for centuries. Therefore, it is classified both as a vegetable and a medicinal herb. It is a fast-growing plant, native to parts of Africa and Asia. It thrives in tropical and sub-tropical climates, making it suitable for worldwide cultivation.[1,2] It is gaining popularity because of its significant health benefits and ease of cultivation.[3,4] In many traditions, it has been incorporated into diets to alleviate stress and increase the energy level. It is also used to promote healthy skin.[5,6] The plant is rich in essential nutrients such as minerals, proteins, vitamins, polyphenols, flavonoids, glucosinolates, isothiocyanates, and alkaloids [1,2]. The bark and roots are used to treat toothaches and stomach aches.[7] The leaves are extensively used for their medicinal properties. Leaves contain anti-inflammatory and antioxidant flavonoids, including myricetin, quercetin, and kaempferol. These phytochemicals have the potential to treat cancer, lipidemia, diabetes, and high blood pressure.[8] It has been documented that moringa can be used as an antifungal, antibacterial, diuretic, testosterone stimulant, for sore throat, and for influenza symptoms.[5,6] Recently, studies have highlighted its therapeutic potential to treat diabetes due to its hypoglycemic effects in diabetic animal models and also showed its capacity to combat oxidative stress and inflammation.[9,10,11]
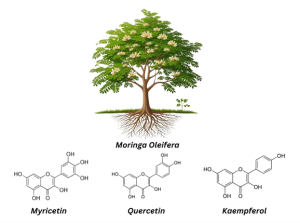
Figure 1: Moringa oleifera and its important phytochemicals
Nutritional Composition: Nutritionally, Moringa has been a well-known plant for centuries, mainly its leaves, which consist of essential vitamins and minerals. Research shows that Moringa leaves are particularly rich in:
- Vitamins: The leaves are a great source of vitamins A, C, and E, which are important for promoting healthy vision, skin, and immune system function.[12]
- Minerals: It provides minerals such as calcium, potassium, iron, and magnesium, which help maintain various physiological functions.[13]
- Proteins: Moringa leaves contain approximately 25% protein content. So, it serves as an excellent source of plant-based protein.[12]
Phytochemical composition: Moringa oleifera contains different types of phytochemicals, including:
- Flavonoids: They are known for their antioxidant properties, which help to reduce oxidative stress and inflammation.[14]
- Glucosinolates: It is available in leaves and seeds. It is known for having potential anticancer properties.[15]
- Tannins and Saponins: These phytochemicals contribute to antimicrobial activities and exhibit anti-inflammatory effects.[14,16]
| Medicinal Properties | Description |
| Antioxidant activity | Antioxidants, such as quercetin and kaempferol present in leaves helps to neutralize free radicals, protecting cells from oxidative stress and lower the risk of chronic diseases.[17,18] |
| Anti-inflammatory effects | Moringa leaves extracts reduces inflammation by inhibiting mediators involved in inflammatory processes, thus promoting recovery.[19,20] |
| Antimicrobial activity | Moringa leaf extracts demonstrate antimicrobial effects against various pathogens, including bacteria and fungi.[19,21] |
| Antidiabetic effects | Moringa leaves have been shown potential to decrease blood sugar levels and improve glucose tolerance, thus indicating its use in managing diabetes.[22,23] |
| Antihypertensive effects | Moringa can help lower blood pressure by improving vascular function and reducing oxidative stress.[22] |
| Anticancer potential | Moringa extracts have been studied for their ability to inhibit the growth of cancer cells and induce apoptosis, offering promise in cancer treatment.[15,17,24] |
Table 1: Medicinal properties of Moringa oleifera
| Aspect | Key points |
| Dyslipidemia in Type II Diabetes | Type II Diabetes patients often have excessive lipid accumulation. Lipids are prone to oxidative damage by free radicals, leading to lipid peroxidation.[25,26] |
| Oxidative stress | Caused by an imbalance between reactive oxygen species (ROS)/ reactive nitrogen species (RNS) production and antioxidant defenses.[27,28] |
| Common ROS include hydroxyl radical (•OH), superoxide anion (O2•-), and peroxynitrite (ONOO−).[29,30] | |
| Free radicals damage lipids, proteins, and nucleic acids, causing impaired signaling and inflammation.[31] | |
| Antioxidant deficiency | Type II Diabetes patients exhibit reduced antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and HO-1.[32,33] |
| Dietary antioxidants | Compounds like salvianolic acid, aspalathin, and resveratrol are being evaluated to enhance conventional treatments like metformin.[34,35,36] |
| Metformin in Type II diabetes | Metformin lowers glucose, lipid levels, and body weight, partly by activating the adenosine monophosphate-activated protein kinase (AMPK) pathway.[37] |
| Thiazolidinediones | Improve insulin sensitivity by activating peroxisome proliferator-activated receptor gamma (PPARγ), reducing fatty acids in peripheral tissues.[37] |
| Side effects of drugs | Both metformin and thiazolidinediones have side effects, and their long-term efficacy is uncertain.[38] |
| Role of medicinal plants | Moringa oleifera shows potential in improving metabolic function due to its antioxidant and anti-inflammatory properties.[39,40] |
| Medicinal plants activate nuclear factor erythroid 2–related factor 2 (Nrf2), aiding cellular detoxification and reducing oxidative stress and inflammation.[41] |
Table 2: Various aspects of type II diabetes that have been studied
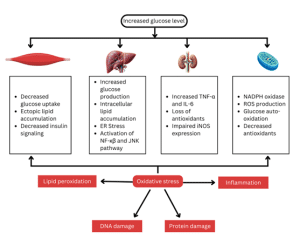
Figure 2: Pathological mechanisms of diabetes mellitus
The pathological mechanisms involved in diabetes mellitus development are complex and multifaceted (Figure 2). Overnutrition (Characterized by increased adipocyte size) and hyperglycemia (consistent increased levels of glucose) can lead to metabolic complications in major organs like the skeletal muscle, liver, and kidneys. This is characterized by impaired glucose homeostasis/insulin signaling, lipid accumulation, mitochondrial dysfunction, endoplasmic reticulum stress, decreased antioxidant responses, increased ROS production, and altered actions of inducible nitric oxide synthase and lipid peroxidation/deoxyribonucleic acid (DNA) damage. This can also result in raised pro-inflammatory markers like tumor necrosis factor-alpha (TNF-α), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κβ), c-jun n-terminal kinase (JNK), and interleukin-6 (IL-6).
Methodology
Experimental studies
Experimental studies on Moringa oleifera extracts in diabetic animal models
According to a study conducted by Jaiswal et al.[42], Moringa oleifera extracts are effective in reducing oxidative stress in Wistar rats induced with streptozotocin (STZ). The extract contains 200 mg/kg of Moringa oleifera leaf, which, in administration for three weeks, demonstrated increased antioxidant activities of SOD, glutathione S-transferase (GST), and CAT, and decreased lipid peroxide levels. Yassa and Tohamy (2014) also used STZ-induced diabetic Sprague Dawley rats, treating them with 200 mg/kg Moringa oleifera extract for eight weeks. They observed lowered fasting plasma glucose (FPG) and significant pancreatic protection, indicated by enhanced glutathione (GSH) levels and reduced malondialdehyde (MDA) concentrations.[43]
In a study conducted by Al-Malki El Rabey, it was found that albino rats that were induced with STZ on treatment with 50 and 100 mg/kg of Moringa oleifera seed extract for four weeks showed decreased fasting plasma glucose (FPG) levels, increased serum and kidney antioxidant levels, particularly SOD and CAT, and reduced IL-6 and lipid peroxides.[44] Muhammad et al.[45] explored that Moringa oleifera leaf extracts have wound healing properties. They revealed that STZ-nicotinamide-induced diabetic Wistar rats on treatment with varying concentrations of 0.5%, 1%, and 2% w/w Moringa extracts for three weeks showed significant decreased wound size, improved tissue regeneration, and reduced inflammatory mediators.
The study of Tuorkey’s found that 100 mg/kg Moringa oleifera aqueous extract reduced fasting plasma glucose (FPG) and plasma insulin levels in alloxan-induced diabetic albino mice, enhanced antioxidant capacity, and reduced creatinine and urea levels on treatment for over two weeks.[46] In research conducted by Abd Eldaim et al, it was shown that 250 mg/kg of Moringa oleifera leaf extract prevented hepatic damage, normalized GSH levels, increased SOD and CAT activities, and reduced blood glucose and hepatic lipid peroxidation in alloxan-induced diabetic Wistar rats when treated for 2.5 weeks with extracts.[47]
Omodanisi et al.[48] a study found that using 250 mg/kg of Moringa oleifera extract on STZ-induced diabetic Wistar rats for six weeks reduced hepatic enzyme markers, normalized lipid profiles, and enhanced antioxidant capacity. Another study by Omodanisi et al.[49] found that FPG and oxidative stress biomarkers decreased, while antioxidant activity increased. The study by Raafat and Hdaib found that 250 mg/kg of Moringa oleifera leaf extract reduced FPG and lipid peroxidation while increasing glycogen synthase gene expression in alloxan-induced diabetic albino rats in 2.5 weeks.[50]
Alejandra Sánchez-Muñoz et al.[51] studied the effects of 200 mg/kg of Moringa oleifera leaf extract in STZ-induced diabetic Wistar rats over three weeks. They found that the leaf extract improved oxidative stress parameters in liver mitochondria and increased antioxidant levels in STZ-induced diabetic Wistar rats. Azevedo et al.[52] reported that 100 mg/kg of Moringa oleifera leaf extract treatment improved wound healing and reduced glycemia in STZ-induced diabetic rats. The research conducted by Oboh et al.[53] found that treating STZ-induced diabetic Wistar rats with 2% and 4% Moringa oleifera leaf and seed extracts for two weeks reduced FPG levels and improved antioxidant activity, potentially preventing cognitive decline. Aju et al.[54] found that treating STZ-induced diabetic Sprague-Dawley rats with 300 mg/kg of Moringa oleifera leaf extract for 8.5 weeks reduced FPG and HbA1C levels, enhanced plasma insulin levels, and enhanced antioxidant enzyme activities.
Oguntibeju et al.[55] conducted a study with Wistar rats treated with 250 mg/kg Moringa oleifera leaf extract for six weeks, revealing a reduction in nephrotoxic and hepatotoxic damage alongside decreased serum creatinine and inflammatory cytokines. Sierra-Campos et al.[56] showed that 200 mg/kg of Moringa oleifera leaf extract in alloxan-induced diabetic Wistar rats increased serum paraoxonase 1 levels and liver cytosolic CAT, further supporting its antidiabetic potential. Finally, Oldoni et al.[57] reported that a crude leaf extract at 500 mg/kg reduced FPG and protected against oxidative damage in the liver and kidney, enhancing antioxidant defenses while decreasing lipid peroxidation. Oyeleye et al.[58] studied the reversal of erectile dysfunction in STZ-induced diabetic Wistar rats using 2% and 4% Moringa oleifera leaf and seed extracts for two weeks, finding improved nitric oxide levels and decreased thiobarbituric acid reactive species (TBARS) levels.
Studies on Moringa oleifera extracts in preclinical models of type II diabetes
Waterman et al.[59] demonstrated that a 5% Moringa oleifera concentrate (delivering 66 mg/kg/d of moringa isothiocyanates) treatment in high-fat diet-fed (HFD) C57BL/6L mice showed improved glucose tolerance and prevented fatty liver disease. It also exhibited plasma levels of insulin, leptin, resistin, cholesterol, IL-1β, and TNF-α, while lowering hepatic glucose-6-phosphatase expression. In another study by Joung et al.[60], it was found that treating C57BL/6 mice with 250 mg/kg Moringa oleifera leaf extract for 10 weeks reduced hepatic lipid accumulation and HFD-induced glucose intolerance while also improving energy metabolism-related gene expression and altering inflammatory markers like uncoupling protein 2/3, TNF-α, IL-1β, IL-6, and MCP-1. Tang et al.[61] found that 150 mg/kg Moringa oleifera leaf ethanolic extract on administration to type II diabetic mice resulted in reduced fasting plasma glucose levels, increased insulin levels, improved lipid profiles, and protected against renal damage by reducing pro-inflammatory markers.
Moreover, a study by Jaja-Chimedza et al.[62] observed that HFD-induced obese C57BL/6J mice, when treated with Moringa oleifera seed extract for 12 weeks, showed reduced body weight, improved glucose tolerance, and altered inflammatory and antioxidant gene expressions, with decreased IL-1β and IL-6 levels and increased inducible nitric oxide synthase (iNOS) expression.
Chin et al.[63] studied the wound healing properties of Moringa oleifera in HFD and streptozotocin-induced diabetic Sprague Dawley rats, finding significant improvements in healing due to modulation of pro-inflammatory markers and growth factors, including TNF-α and vascular endothelial growth factor (VEGF). In a related study, Mohamed et al.[64] found that treating HFD-induced insulin-resistant Sprague Dawley rats with Moringa oleifera aqueous extract for 4 weeks reversed hepatic insulin insensitivity, up-regulated insulin receptor genes, enhanced hepatic antioxidants like catalase and superoxide dismutase, and decreased lipid peroxidation levels.
Finally, El-Shehawi et al.[65] reported that HFD-induced obese Wistar rats treated with 300 mg/kg Moringa oleifera leaf extract for 14 weeks exhibited reduced body weight and fat mass, along with lower FPG, insulin, and leptin levels. The treatment also increased adiponectin and improved lipid profiles, enhancing hepatic antioxidant enzymes while decreasing lipid peroxidation and pro-inflammatory markers.
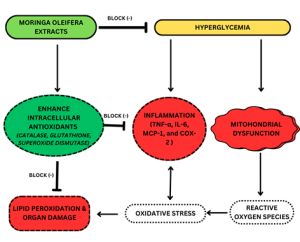
Figure 3: Therapeutic effects of Moringa oleifera extract
Figure 3 shows the therapeutic effects of the extract in preclinical animal models of diabetes. These extracts enhance intracellular antioxidants like catalase, glutathione, and superoxide dismutase, blocking reactive oxygen species, lipid peroxidation, and organ damage. They also improve glucose control and reduce pro-inflammatory markers like TNF-α, IL-6, MCP-1, and COX-2.
Discussion
Future perspective of Moringa oleifera
Future research on Moringa oleifera should focus on
- Conducting robust clinical trials to validate its efficacy and safety in managing diabetes.
- Exploring standardized dosages and formulations for enhanced clinical application.
- Investigating synergistic effects with conventional therapies and assessing long-term impacts on diabetic complications.
- Understanding mechanisms of beneficial effects to identify novel therapeutic targets in diabetes management.
Conclusion
This review article highlights the therapeutic potential of Moringa oleifera extracts in type II diabetes. The evidence from preclinical studies shows that Moringa extracts modulate metabolic parameters, enhance antioxidant defenses, and improve insulin sensitivity, making it a valuable adjunct in diabetes management. It also has positive effects on body weight and wound healing. However, the current research has limitations, including variations in study designs, dosages, and animal models. Future research should focus on understanding the mechanisms of action, optimizing dosages, and conducting clinical trials to confirm these benefits in human populations. In conclusion, Moringa oleifera presents a promising avenue for developing novel therapeutic strategies against Type II diabetes, improving management and quality of life.
References
- Gopalakrishnan L, Doriya K, Kumar DS. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci Hum Wellness. 2016;5(2):49-56. doi:10.1016/j.fshw.2016.04.001 Crossref | Google Scholar
- Trigo C, Castelló ML, Ortolá MD, García-Mares FJ, Desamparados Soriano M. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods. 2020;10(1):31. doi:10.3390/foods10010031 PubMed | Crossref | Google Scholar
- Alegbeleye OO. How Functional Is Moringa oleifera? A Review of Its Nutritive, Medicinal, and Socioeconomic Potential. Food Nutr Bull. 2018;39(1):149-170. doi:10.1177/0379572117749814 PubMed | Crossref | Google Scholar
- Zhu Y, Yin Q, Yang Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules. 2020;25(3):676. doi:10.3390/molecules25030676 PubMed | Crossref | Google Scholar
- Mishra G, Singh P, Verma R, Kumar S, Srivastav S, Jha K, Khosa RL. Traditional uses, phytochemistry, and pharmacological properties of Moringa oleifera plant: An overview. Der Pharmacia Lettre. 2011;3:141-164. (PDF) Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: An overview
- Kumar RPS, Arts K, Veluswamy B, Malayaman V. Phytochemical screening of aqueous leaf extract of Sida acuta Burm. F. and its antibacterial activity. J Emerg Technol Innov Res. 2018;5:472-478. (PDF) Phytochemical Screening of Aqueous Leaf Extract of Sida acuta Burm. F. and its Antibacterial Activity
- Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int J Mol Sci. 2015;16(6):12791-12835. doi:10.3390/ijms160612791 PubMed | Crossref | Google Scholar
- Vergara-Jimenez M, Almatrafi MM, Fernandez ML. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants (Basel). 2017;6(4):91. doi:10.3390/antiox6040091 PubMed | Crossref | Google Scholar
- Balakrishnan BB, Krishnasamy K, Mayakrishnan V, Selvaraj A. Moringa concanensis Nimmo extracts ameliorates hyperglycemia-mediated oxidative stress and upregulates PPARγ and GLUT4 gene expression in liver and pancreas of streptozotocin-nicotinamide induced diabetic rats. Biomed Pharmacother. 2019;112:108688. doi:10.1016/j.biopha.2019.108688 PubMed | Crossref | Google Scholar
- Chin CY, Ng PY, Ng SF. Moringa oleifera standardised aqueous leaf extract-loaded hydrocolloid film dressing: in vivo dermal safety and wound healing evaluation in STZ/HFD diabetic rat model. Drug Deliv Transl Res. 2019;9(2):453-468. doi:10.1007/s13346-018-0510-z PubMed | Crossref | Google Scholar
- Bao Y, Xiao J, Weng Z, Lu X, Shen X, Wang F. A phenolic glycoside from Moringa oleifera Lam. improves the carbohydrate and lipid metabolisms through AMPK in db/db mice. Food Chem. 2020;311:125948. doi:10.1016/j.foodchem.2019.125948 PubMed | Crossref | Google Scholar
- Gandji K, Chadare FJ, Idohou R, Salako VK, Assogbadjo AE, Glèlè RLK. Status and utilisation of Moringa oleifera Lam: A review. Afr Crop Sci J. 2018;26:137-156. doi:10.4314/acsj.v26i1.10 Crossref | Google Scholar
- Kasolo JN, Bimenya GS, Ojok L, Ochieng J, Ogwal-Okeng JW. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plants Res. 2010;4:753-757. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities
- Chaudhary K, Chourasia S. Nutraceutical properties of Moringa oleifera: A review. Eur J Pharm Med Res. 2017;4:646-655. (PDF) Neutraceutical Properties of Moringa oleifera: A Review
- Guevara AP, Vargas C, Sakurai H, et al. An antitumor promoter from Moringa oleifera Lam. Mutat Res. 1999;440(2):181-188. doi:10.1016/s1383-5718(99)00025-x PubMed | Crossref | Google Scholar
- Popoola JO, Obembe OO. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J Ethnopharmacol. 2013;150(2):682-691. doi:10.1016/j.jep.2013.09.043 PubMed | Crossref | Google Scholar
- Singh A, Navneet. Ethnomedicinal, pharmacological, and antimicrobial aspects of Moringa oleifera Lam.: A review. J Phytopharmacol. 2018;7:45-50. doi:10.31254/phyto.2018.7110 Crossref
- Mishra G, Singh P, Verma R, et al. Traditional uses, phytochemistry, and pharmacological properties of Moringa oleifera plant: An overview. Der Pharm Lett. 2011;3(2):141-164. Traditional uses, phytochemistry, and pharmacological properties of Moringa oleifera plant: An overview
- Gopinath LR, Jeevitha S, Gokiladevi T, Archaya S. Isolation and identification of therapeutic compounds from Moringa oleifera and its antimicrobial activity. IOSR J Pharm Biol Sci. 2017;12(6):1-7. Isolation and identification of therapeutic compounds from Moringa oleifera and its antimicrobial activity
- Stohs SJ, Hartman MJ. Review of the Safety and Efficacy of Moringa oleifera. Phytother Res. 2015;29(6):796-804. doi:10.1002/ptr.5325 PubMed | Crossref | Google Scholar
- Tayo GM, Poné JW, Komtangi MC, et al. Anthelmintic activity of Moringa oleifera leaf extracts evaluated in vitro on four developmental stages of Haemonchus contortus from goats. Am J Plant Sci. 2014;2014. doi:10.4236/ajps.2014.511185 Crossref | Google Scholar
- Aekthammarat D, Pannangpetch P, Tangsucharit P. Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine. 2019;54:9-16. doi:10.1016/j.phymed.2018.10.023 PubMed | Crossref | Google Scholar
- Singh A, Navneet. Ethnomedicinal, pharmacological, and antimicrobial aspects of Moringa oleifera Lam.: A review. J Phytopharmacol. 2018;7:45-50. doi:10.31254/phyto.2018.7110 Crossref
- Paikra BK, Dhongade HKJ, Gidwani B. Phytochemistry and Pharmacology of Moringa oleifera Lam. J Pharmacopuncture. 2017;20(3):194-200. doi:10.3831/KPI.2017.20.022 PubMed | Crossref | Google Scholar
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71(5):551-564. doi:10.1016/j.bcp.2005.10.044 PubMed | Crossref | Google Scholar
- Ito F, Sono Y, Ito T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants (Basel). 2019;8(3):72. doi:10.3390/antiox8030072 PubMed | Crossref | Google Scholar
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058-1070. doi:10.1161/CIRCRESAHA.110.223545 PubMed | Crossref | Google Scholar
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126-1167. doi:10.1089/ars.2012.5149 PubMed | Crossref | Google Scholar
- Burgos-Morón E, Abad-Jiménez Z, Marañón AM, et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J Clin Med. 2019;8(9):1385. doi:10.3390/jcm8091385 PubMed | Crossref | Google Scholar
- Chandra K, Singh P, Dwivedi S, Jain SK. Diabetes mellitus and oxidative stress: A co-relative and therapeutic approach. J Clin Diagn Res. 2019;13(5):7-12. doi:10.7860/JCDR/2019/40628.12878 Crossref | Google Scholar
- Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5-18. doi:10.12816/0003082 PubMed | Crossref | Google Scholar
- Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Botany. 2012;2012:217037. doi:10.1155/2012/217037 Crossref |
Google Scholar - Kim JH, Cho JH, Kim SR, Hur YB. Toxic effects of waterborne ammonia exposure on hematological parameters, oxidative stress, and stress indicators of juvenile hybrid grouper, Epinephelus lanceolatus × Epinephelus fuscoguttatus. Environ Toxicol Pharmacol. 2020;80:103453. doi:10.1016/j.etap.2020.103453 PubMed | Crossref |
Google Scholar - Frendo-Cumbo S, MacPherson RE, Wright DC. Beneficial effects of combined resveratrol and metformin therapy in treating diet-induced insulin resistance. Physiol Rep. 2016;4(15):e12877. doi:10.14814/phy2.12877 PubMed | Crossref |
Google Scholar - Wu P, Yan Y, Ma LL, et al. Effects of the Nrf2 Protein Modulator Salvianolic Acid A Alone or Combined with Metformin on Diabetes-associated Macrovascular and Renal Injury. J Biol Chem. 2016;291(42):22288-22301. doi:10.1074/jbc.M115.712703 PubMed | Crossref | Google Scholar
- Dludla PV, Gabuza KB, Muller CJF, Joubert E, Louw J, Johnson R. Aspalathin, a C-glucosyl dihydrochalcone from rooibos improves the hypoglycemic potential of metformin in type 2 diabetic (db/db) mice. Physiol Res. 2018;67(5):813-818. doi:10.33549/physiolres.933891 PubMed | Crossref | Google Scholar
- Greenfield JR, Chisholm DJ. Thiazolidinediones: Mechanisms of action. Aust Prescr. 2004;27:67-70. doi:10.18773/austprescr.2004.059 Crossref | Google Scholar
- DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism. 2016;65(2):20-29. doi:10.1016/j.metabol.2015.10.014 PubMed | Crossref | Google Scholar
- Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights?. Diabetologia. 2013;56(9):1898-1906. doi:10.1007/s00125-013-2991-0 PubMed | Crossref | Google Scholar
- Yendapally R, Sikazwe D, Kim SS, et al. A review of phenformin, metformin, and imeglimin. Drug Dev Res. 2020;81(4):390-401. doi:10.1002/ddr.21636 PubMed | Crossref | Google Scholar
- Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401-426. doi:10.1146/annurev-pharmtox-011112-140320 PubMed | Crossref | Google Scholar
- Jaiswal D, Rai PK, Mehta S, et al. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med. 2013;6(6):426-432. doi:10.1016/S1995-7645(13)60068-1 PubMed | Crossref | Google Scholar
- Yassa HD, Tohamy AF. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced Diabetes mellitus in adult rats. Acta Histochem. 2014;116(5):844-854. doi:10.1016/j.acthis.2014.02.002 PubMed | Crossref | Google Scholar
- Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed Res Int. 2015;2015:381040. doi:10.1155/2015/381040 PubMed | Crossref | Google Scholar
- Muhammad AA, Pauzi NA, Arulselvan P, Abas F, Fakurazi S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. Biomed Res Int. 2013;2013:974580. doi:10.1155/2013/974580 PubMed | Crossref | Google Scholar
- Tuorkey MJ. Effects of Moringa oleifera aqueous leaf extract in alloxan induced diabetic mice. Interv Med Appl Sci. 2016;8(3):109-117. doi:10.1556/1646.8.2016.3.7 PubMed | Crossref | Google Scholar
- Abd Eldaim MA, Shaban Abd Elrasoul A, Abd Elaziz SA. An aqueous extract from Moringa oleifera leaves ameliorates hepatotoxicity in alloxan-induced diabetic rats. Biochem Cell Biol. 2017;95(4):524-530. doi:10.1139/bcb-2016-0256 PubMed | Crossref | Google Scholar
- Omodanisi EI, Aboua YG, Chegou NN, Oguntibeju OO. Hepatoprotective, Antihyperlipidemic, and Anti-inflammatory Activity of Moringa oleifera in Diabetic-induced Damage in Male Wistar Rats. Pharmacognosy Res. 2017;9(2):182-187. doi:10.4103/0974-8490.204651 PubMed | Google Scholar
- Omodanisi EI, Aboua YG, Oguntibeju OO. Assessment of the Anti-Hyperglycaemic, Anti-Inflammatory and Antioxidant Activities of the Methanol Extract of Moringa Oleifera in Diabetes-Induced Nephrotoxic Male Wistar Rats. Molecules. 2017;22(4):439. doi:10.3390/molecules22040439 PubMed | Crossref | Google Scholar
- Raafat K, Hdaib F. Neuroprotective effects of Moringa oleifera: Bio-guided GC-MS identification of active compounds in diabetic neuropathic pain model. Chin J Integr Med. 2017:1-10. doi:10.1007/s11655-017-2758-4 PubMed | Crossref | Google Scholar
- Alejandra Sánchez-Muñoz M, Valdez-Solana MA, Campos-Almazán MI, et al. Streptozotocin-Induced Adaptive Modification of Mitochondrial Supercomplexes in Liver of Wistar Rats and the Protective Effect of Moringa oleifera Lam. Biochem Res Int. 2018;2018:5681081. doi:10.1155/2018/5681081 PubMed | Crossref | Google Scholar
- Azevedo ÍM, Araújo-Filho I, Teixeira MMA, Moreira MDFC, Medeiros AC. Wound healing of diabetic rats treated with Moringa oleifera extract. Acta Cir Bras. 2018;33(9):799-805. doi:10.1590/s0102-865020180090000008 PubMed | Crossref | Google Scholar
- Oboh G, Oyeleye SI, Akintemi OA, Olasehinde TA. Moringa oleifera supplemented diet modulates nootropic-related biomolecules in the brain of STZ-induced diabetic rats treated with acarbose. Metab Brain Dis. 2018;33(2):457-466. doi:10.1007/s11011-018-0198-2 PubMed | Crossref | Google Scholar
- Aju BY, Rajalakshmi R, Mini S. Protective role of Moringa oleifera leaf extract on cardiac antioxidant status and lipid peroxidation in streptozotocin induced diabetic rats. Heliyon. 2019;5(12):e02935. doi:10.1016/j.heliyon.2019.e02935 PubMed | Crossref | Google Scholar
- Oguntibeju OO, Aboua GY, Omodanisi EI. Effects of Moringa oleifera on oxidative stress, apoptotic, and inflammatory biomarkers in streptozotocin-induced diabetic animal model. South Afr J Bot. 2020;129:354 365. doi:10.1016/j.sajb.2019.08.039 Crossref | Google Scholar
- Sierra-Campos E, Valdez-Solana M, Avitia-Domínguez C, et al. Effects of Moringa oleifera Leaf Extract on Diabetes-Induced Alterations in Paraoxonase 1 and Catalase in Rats Analyzed through Progress Kinetic and Blind Docking. Antioxidants (Basel). 2020;9(9):840. doi:10.3390/antiox9090840 PubMed | Crossref | Google Scholar
- Oldoni TLC, Merlin N, Bicas TC, et al. Antihyperglycemic activity of crude extract and isolation of phenolic compounds with antioxidant activity from Moringa oleifera Lam. leaves grown in Southern Brazil. Food Res Int. 2021;141:110082. doi:10.1016/j.foodres.2020.110082 PubMed | Crossref | Google Scholar
- Oyeleye SI, Ojo OR, Oboh G. Moringa oleifera leaf and seed inclusive diets influenced the restoration of biochemicals associated with erectile dysfunction in the penile tissue of STZ-induced diabetic male rats treated with/without Acarbose drug. J Food Biochem. 2021;45(3):e13323. doi:10.1111/jfbc.13323 PubMed | Crossref | Google Scholar
- Waterman C, Rojas-Silva P, Tumer TB, et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol Nutr Food Res. 2015;59(6):1013-1024. doi:10.1002/mnfr.201400679 PubMed | Crossref | Google Scholar
- Joung H, Kim B, Park H, et al. Fermented Moringa oleifera Decreases Hepatic Adiposity and Ameliorates Glucose Intolerance in High-Fat Diet-Induced Obese Mice. J Med Food. 2017;20(5):439-447. doi:10.1089/jmf.2016.3860 PubMed | Crossref | Google Scholar
- Tang Y, Choi EJ, Han WC, et al. Moringa oleifera from Cambodia Ameliorates Oxidative Stress, Hyperglycemia, and Kidney Dysfunction in Type 2 Diabetic Mice. J Med Food. 2017;20(5):502-510. doi:10.1089/jmf.2016.3792 PubMed | Crossref | Google Scholar
- Jaja-Chimedza A, Zhang L, Wolff K, et al. A dietary isothiocyanate-enriched moringa (Moringa oleifera) seed extract improves glucose tolerance in a high-fat-diet mouse model and modulates the gut microbiome. J Funct Foods. 2018;47:376-385. doi:10.1016/j.jff.2018.05.056 PubMed | Crossref | Google Scholar
- Chin CY, Ng PY, Ng SF. Moringa oleifera standardised aqueous leaf extract-loaded hydrocolloid film dressing: in vivo dermal safety and wound healing evaluation in STZ/HFD diabetic rat model. Drug Deliv Transl Res. 2019;9(2):453-468. doi:10.1007/s13346-018-0510-z PubMed | Crossref | Google Scholar
- Mohamed MA, Ahmed MA, El Sayed RA. Molecular effects of Moringa leaf extract on insulin resistance and reproductive function in hyperinsulinemic male rats. J Diabetes Metab Disord. 2019;18(2):487-494. doi:10.1007/s40200-019-00454-7 PubMed | Crossref | Google Scholar
- El-Shehawi AM, Alkafafy M, El-Shazly S, et al. Moringa oleifera leaves ethanolic extract ameliorates high-fat diet-induced obesity in rats. J King Saud Univ Sci. 2021;33(6):101552. doi:10.1016/j.jksus.2021.101552 Crossref | Google Scholar
Acknowledgments
The authors express their heartfelt gratitude to all contributors, including mentors and colleagues, for their invaluable support during the development of this review article. We also extend our appreciation to our families and friends for their unwavering encouragement throughout this journey.
Funding
This review article received no external funding.
Author Information
Corresponding Author:
Sannu Ahmed
Department of Pharmacy
National Academy for Medical Sciences, Old Baneshwor, Kathmandu, Nepal
Email: pr.sannu.ahmed@gmail.com
Co-Authors:
Pradip Regmi
Department of Pharmacy
Nepal Institute of Health Sciences, Jorpati, Kathmandu, Nepal
Sapana Pandey
Department of Pharmacy
Asian College for Advance Studies, Satdobato, Lalitpur, Nepal
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Conflict of Interest Statement
The author declares no conflict of interest.
Guarantor
None
DOI
Cite this Article
Sannu A, Pradip R, Sapana P. Effectiveness of Moringa Oleifera Extract in Type II Diabetes: A Comprehensive Literature Review. medtigo J Pharmacol. 2024;1(2):e3061121. doi:10.63096/medtigo3061121 Crossref









