Author Affiliations
Abstract
The market demand for orally disintegrating tablets has experienced a significant surge over the past decade. This intervention is particularly beneficial for geriatric and pediatric patients who experience challenges in ingesting traditional tablets and capsules. The utilization of a fast-dissolving or fast-disintegrating dosage form confers advantages to patients in this context. Fast dissolvable or fast disintegrating dosage forms are designed to rapidly disintegrate upon exposure to saliva, resulting in accelerated drug release within the oral cavity. The administration of fast-disintegrating dosage forms facilitates the absorption of drugs through the buccal mucosa, potentially resulting in a reduction of first-pass metabolism and consequently enhancing the desired drug efficacy. The objective of this study was to create six different formulations (F1, F2, F3, F4, F5, and F6) of naproxen MDT through the direct compression method, utilizing different concentrations of superdisintegrants. A range of pre-formulation and post formulation evaluation studies were conducted, including compatibility studies, bulk density analysis, tapped density determination, angle of repose assessment, cars index evaluation, Hausner’s ratio, weight variation analysis, hardness assessment, friability evaluation, drug content assessment, wetting time analysis, in vitro dissolution analysis, in-vitro dispersion technique analysis, and water absorption ratio analysis. The drug release in vitro examination of formulation F4, which incorporates crospovidone as a superdisintegrant, demonstrated the highest level of drug release in comparison to the other formulations. A study was conducted to determine the feasibility of preparing MDT with enhanced naproxen dissolution through the direct compression method, incorporating superdisintegrants. This study facilitated comprehension of the impact of formulation processing variables, particularly the super-disintegrant, on the formulation’s drug release profile.
Keywords
Mouth dissolving tablets, Naproxen, Superdisintegrants, Evaluation studies, Direct compression.
Introduction
An ideal dosage regimen in the drug therapy of any disease is the one that immediately attains the desired therapeutic concentration of the drug in plasma (or at the site of action) and maintains it constant for the entire duration of treatment. This is possible through the administration of a conventional dosage form in a particular dose and at a particular frequency.[1] Thus, the drug may be administered by a variety of routes in a variety of dosage forms.
Drugs are more frequently taken by oral administration. Although a few drugs taken orally are intended to be dissolved within the mouth, most drugs taken orally are swallowed. Compared with alternative routes, the oral route of drug administration is the most popular and has been successfully used for the conventional delivery of drugs. It is considered the most natural, uncomplicated, convenient, safe means of administering drugs, with greater flexibility in dosage form design, ease of production, and low cost.[2]
Tablets and hard gelatin capsules constitute a major portion of the drug delivery systems that are currently available. However, many patient groups, such as the elderly, children, and patients with intellectual disability, mental retarted, uncooperative, nausea, or reduced liquid intake diets, have difficulty swallowing these dosage forms. Many elderly people face difficulties in administering conventional oral dosage forms because of hand tremors and dysphasia. Swallowing problems are common in children because of their underdeveloped muscular and nervous systems. In some cases, like motion sickness, sudden episodes of allergic attack or coughing, and during the unavailability of water, swallowing conventional tablets is difficult. To fulfill these medical needs, formulators have devoted considerable efforts to developing a novel type of dosage form for oral administration known as MDT.[3]
MDT is an innovative tablet technology where the dosage form containing active pharmaceutical ingredients disintegrates rapidly, usually in a matter of seconds, without the need for water, providing optimal convenience to the patient. Innovators and inventor companies have given these tablets various names such as orally disintegrating tablets (ODT), mouth dissolving (MD), fast melting, fast dissolving, or Orodisperse. The European Pharmacopoeia defines Orodisperse as a tablet that can be placed in the mouth where it disperses rapidly before swallowing. Researchers have formulated ODT for various categories of drugs, which are used for therapy in which a rapid peak plasma concentration is required to achieve the desired pharmacological response. These include neuroleptics, cardiovascular agents, analgesics, anti-allergic, and drugs for erectile dysfunction.[4,5]
Direct compression represents the simplest and most cost-effective tablet manufacturing technique. This technique can now be applied for the preparation of ODT because of the availability of improved excipients, especially superdisintegrants like croscarmellose sodium, Ac-Di-Sol, crospovidone, Kollidon, Explotab, Primogel, Satialgine, Emcosoy, Calcium silicate, and sugar-based excipients like dextrose, fructose, isomalt, lactilol, maltilol, maltose, mannitol, sorbitol, starch hydrolysate, polydextrose, and xylitol.[6,7]
Basic goals in the development of mouth-dissolving tablets are to increase patient compliance, ease of administration, safety, and appropriate dosing. It has a perceived faster onset of action as the dosage form is disintegrated prior to reaching the stomach. It is ideal for acute diseases like allergies, nausea, and vomiting, and is particularly applicable to managing breakthrough symptoms. Direct compression method was used to compress the tablets as it is the easiest way to manufacture tablets and is less time-consuming. The aim of the present work is to investigate the possibility of preparing a mouth-dissolving tablet containing naproxen by the direct compression method.
Methodology
Materials
Naproxen was obtained as a gift sample from Granules India, Hyderabad, India. Microcrystalline cellulose, mannitol, croscarmellose sodium, crospovidone, sodium starch glycollate, aerosil, aspartame, magnesium stearate from Kniss Laboratories Pvt limited, Chennai.
Methodology
Drug-Excipient Compatibility Studies: The compatibility studies were performed using Fourier Transform- Infra Red (FTIR) spectroscopy. The drug and drug-polymer physical mixtures were scanned in the region of 4000-400 cm-1.
Pre-formulation Studies:[8,9,10]
All the Ingredients were passed through a mesh 60. The required quantity of each ingredient was taken for each specified formulation, and all the ingredients were cog rind in a mortar and pestle. The powder blend was evaluated for flow properties such as bulk density, tapped density, compressibility index, Hausner’s ratio, and angle of repose.
Bulk density: A given quantity of the powder is transferred to the measuring cylinder, and it is tapped mechanically, either manually or with a mechanical device, till a constant volume is obtained. This volume is bulk volume (v), and it includes the true volume of the powder and the void space among the powder particles. It is the ratio between a given mass of powder and its bulk volume.
Bulk density = Mass of powder / Total weight of the powder
Tapped density: Tapped density is defined as the ratio between the weight of the sample powder taken and the tapped volume.
Tapped density (ρt) = M/Vt; M = weight of sample powder taken and Vt = tapped volume
Compressibility index /Carr’s index: Based on the apparent bulk density and the tapped density, the percentage compressibility index of the powder was determined by using the following formula.
Compressibility index = Tapped Density-Bulk density / Tapped density X 100
Hausner ratio: By calculating tapped density and bulk density, the Hausner ratio can be calculated.
Hausner ratio = tapped density/bulk density
Angle of repose: Angle of repose is defined as the maximum angle possible between the surface of a pile of powder and the horizontal plane. The granule mass should be allowed to flow out of the funnel orifice onto a plane paper kept on a horizontal surface. This forms a pile of granules on the paper.
tan θ = h/r
θ = tan-1(h/r) Where, h= height of the pile and r= radius of the pile
Preparation of MDT of Naproxen: Six formulations (F1, F2, F3, F4, F5, and F6) of naproxen MDT are prepared at varying concentrations of superdisintegrants and are formulated by the direct compression method (Table 1). Sift naproxen, microcrystalline cellulose, superdisintegrants, aerosil, aspartame, mannitol, through #40 mesh separately, collect in poly bags. Naproxen, microcrystalline cellulose, superdisintegrants, aerosil, aspartame, and mannitol were co-grinded in a motor and pestle mix for 10 minutes. Finally, magnesium stearate was added and mixed for 10 minutes then sifted through #60 mesh. Tablets are prepared by fixing the tablet machine and compressing using the Rimek Minipress-I tablet punching machine to produce convex-faced tablets weighing 250 mg.
| S. No | Ingredients | F1
(mg) |
F2
(mg) |
F3
(mg) |
F4
(mg) |
F5
(mg) |
F6
(mg) |
| 1. | Naproxen | 150 | 150 | 150 | 150 | 150 | 150 |
| 2. | Microcrystalline Cellulose | 18 | 10 | 18 | 10 | 18 | 10 |
| 3. | Mannitol | 26 | 14 | 26 | 14 | 26 | 14 |
| 4. | Croscarmellose Sodium | 40 | 60 | – | – | – | – |
| 5. | Crospovidone | – | – | 40 | 60 | – | – |
| 6. | Sodium Starch Glycolate | – | – | – | – | 40 | 60 |
| 7. | Aerosil | 2 | 2 | 2 | 2 | 2 | 2 |
| 8. | Aspartame | 10 | 10 | 10 | 10 | 10 | 10 |
| 9. | Magnesium stearate | 4 | 4 | 4 | 4 | 4 | 4 |
| Average Weight | 250 | 250 | 250 | 250 | 250 | 250 | |
Table 1: Naproxen MDT
Evaluation of MDT[11,12,13,14,15]
Thickness: The thickness of the tablets was determined by using micrometer screw gauge and the results were expressed in millimeters. A ± 5% may be allowed depending on the size of the tablet.
Weight variation test: Ten tablets were selected at random, individually weighed in a single pan electronic balance, and the average weight was calculated. The uniformity of weight was determined according to the Indian Pharmacopoeia (IP) specification. As per IP, not more than two individuals weights would deviate from the average weight by not more than 5%, and none would deviate by more than twice that percentage.
| S.No. | Average weight of tablet | Percentage |
| 1 | 80 mg or less | ± 10% |
| 2 | More than 80mg and less than 250mg | ± 7.5% |
| 3 | 250 mg or more | ± 5% |
Table 2: Limits of weight variation
Hardness test: Tablets crushing load, which is the force required to break a tablet by compression in the radial direction, was determined by using c Monsanto hardness tester. Ten tablets from the batch were used for hardness studies, and results are expressed in Kg/cm2.
Friability test: It was performed in the Roche friabilator apparatus, where the tablets were subjected to the combined effect of abrasion and shock by utilizing a plastic chamber that revolves at 25 rpm, dropping the tablets at six inches with each revolution. Pre-weighed samples of 20 tablets were placed in the friabilator, which was then operated for 100 revolutions. The tablets are then dusted and reweighed. Conventional compressed tablets that lose less than 0.5 to 1% of their weight are generally considered acceptable.
%Friability: Weight loss / Weight of tablets before operation × 100
Disintegration: A USP device consists of six glass tubes that are 3 inches long, open at one end, and held against 10 mesh screens at the bottom end of the basket rack assembly. To test for disintegration time, one tablet is placed in each tube and the basket arch is positioned in 1 liter beaker of water at 37±2°C. A standard motor-driven device is used to move the basket assembly up and down.
Water absorption Ratio: A piece of tissue paper folded twice was placed in a small Petri dish containing 6 ml of water. A tablet was put on the paper & the time required for complete wetting was measured. The wet tablet was then weighed.
Water absorption Ratio = Initial weight / Final weight x 100
Content uniformity test: Ten tablets were weighed and pulverized to a fine powder equivalent to 10mg of naproxen, which was extracted into distilled water, and the liquid was filtered. The naproxen content was determined by measuring the absorbance at 331nm (using a UV-Visible spectrophotometer, Shimadzu) after appropriate dilution with distilled water. The drug content was determined by using a standard calibration curve.
Wetting time: Wetting time is closely related to the inner structure of the tablets and to the hydrophilicity of the excipient. The initial process in the disintegration of an ODT involves water uptake and wetting of the tablet. So, determining the wetting time is also important. It also helps in studying the effect of various excipients on the disintegration of the tablet. A Petri dish containing 6 ml of distilled water was taken, and a tissue paper folded twice was placed in it. A tablet containing a small quantity of amaranth color was placed on this. Time required for the upper surface of the tablet to become completely red is the wetting time, and it was recorded.
In-vitro dispersion time: The test was performed by placing two tablets in 100 ml of water and stirring them gently till the tablets completely disintegrated. The formulation is considered to form a smooth dispersion if the complete dispersion passes through a sieve screen with a nominal mesh aperture of 710 μm without leaving any residue on the mesh. Tablets were added to 100 ml of phosphate buffer solution, pH 6.8, at 37±0.5ºC, Time required for complete dispersion of a tablet was measured.
In vitro dissolution studies: Prepare the standard curve of naproxen (100 mg) in phosphate buffer pH 6.8 using concentrations of 10 μg/ml, 20 μg/ml, 30 μg/ml, 40 μg/ml, 50 μg/ml at 331 nm UV-Visible Spectrophotometer. Tablet dissolution was assessed using a standard USP dissolution apparatus type II. The dissolution media used was 900 ml of 6.8 phosphate buffer. Maintained parameters like temperature 37±0.5ºC, paddle speed 50 rpm, total sampling time 60min. At predetermined time intervals, an aliquot of 5 ml of the sample was withdrawn and made up to 10 ml with buffer.
Results
Physical parameters:
| S. No | Parameters | Result |
| 1. | Tapped density | 0.728 gm/ml |
| 2. | Bulk density | 0.521 gm/ml |
| 3. | Compressibility Index | 28.40 % |
| 4. | Hausner Ratio | 1.397 |
| 5. | Angle of repose | 350311 |
Table 3: Physical parameters of API in preformulation studies
The pure drug showed an angle of repose value of 350311indicates good flow properties. The compressibility index, Hausner’s ratio values of the drug are 28.40% and 1.397, indicating that the drug has poor compressibility properties.
Drug–Excipient compatibility studies
FTIR: The FTIR analysis was conducted for the structure characterization. FTIR spectra of the pure drug, pure polymers, and a mixture of both were recorded. Formulations were taken in a KBr pellet using a BOMEN MB SERIES FTIR instrument. Approximately 5mg of samples were mixed with 50mg of spectroscopic grade KBr; samples were scanned in the IR range from 500 to 3500 cm-1, with a resolution of 4 cm-1. The drug excipient compatibility study showed no interactions as principal peaks are retained. Thus, all excipients were compatible with the drug. The FTIR of the drug alone and when combined with superdisintegrants is shown in Figures 1 and 2.
Figure 1: FTIR of Naproxen
Figure 2: FTIR of naproxen with sodium starch glycolate
Precompression Properties:
| Formulation | Bulk density
(g/ml) |
Tapped density
(g/ml) |
Hausner’s ratio | Carr’s index | Angle of repose
(0o) |
| F1 | 0.53±0.005 | 0.62±0.02 | 1.16±0.02 | 13.65±1.06 | 31′ |
| F2 | 0.52±0.01 | 0.61±0.014 | 1.18±0.03 | 14.27±1.06 | 30º. |
| F3 | 0.526±0.005 | 0.61±0.014 | 1.16±0.01 | 14.78±0.18 | 27º9′ |
| F4 | 0.52±0 | 0.60 | 1.15±0 | 14.58±0 | 32º.05′ |
| F5 | 0.526±0.01 | 0.605±0.01 | 1.163±0.02 | 12.51±0.02 | 29º.82′ |
| F6 | 0.526±0.005 | 0.63±0.08 | 1.163±0.005 | 14.89±0 | 27º.21′ |
Table 4: Precompression properties of the naproxen mouth dissolving tablets
The data are expressed as mean±SD(n=3)
Post compression properties:
| Formulation | Weight variation (mg) | Thickness (mm) | Hardness (kg/cm2)
|
Friability (%) | Disintegration Time (sec) |
| F1 | Passes | 3.74±0.02 | 3.63±0.15 | 0.50 | 28±0.54 |
| F2 | Passes | 3.76±0.02 | 3.8±0.1 | 0.45±0.12 | 26±0.02 |
| F3 | Passes | 3.26±0.02 | 4.03±0.05 | 0.41±0.22 | 25±0.14 |
| F4 | Passes | 3.14±0.02 | 3.33±0.11 | 0.22±0.08 | 26±0.25 |
| F5 | Passes | 3.21±0.03 | 3.8±0.1 | 0.8±0.08 | 27±0.14 |
| F6 | Passes | 3.66±0.05 | 4.03±0.15 | 0.62±0.16 | 24±0.01 |
Table 5: Post-compression properties of naproxen mouth dissolving tablets
The data are expressed as mean±SD(n=3)
| Formulation | Water absorption Ratio | Content uniformity | Wetting time
(sec) |
In-vitro dispersion time (Sec) |
| F1 | 78.92±0.14 | 98.19±0.51 | 50.66±3.05 | 114.66±4.16 |
| F2 | 72.35±0.41 | 98.42±1.01 | 43.33±3.05 | 93.3±4.16 |
| F3 | 69.32±0.58 | 97.77±1.26 | 26±2 | 74.3±4.04 |
| F4 | 75.63±0.47 | 99.82±0.33 | 18±2 | 55±1.0 |
| F5 | 74.21±0.25 | 99.74±0.44 | 72.66±3.05 | 93.6±2.51 |
| F6 | 74.23±0.14 | 98.50±0.55 | 67.33±3.05 | 84±2 |
Table 6: post compression properties of the naproxen mouth dissolving tablets
The data are expressed as mean±SD(n=3)
Invitro drug release:
| S.No | Concentration(µg/ml) | Absorbance at 331 nm |
| 1 | 10 | 0.037 |
| 2 | 20 | 0.0990 |
| 3 | 30 | 0.15 |
| 4 | 40 | 0.206 |
| 5 | 50 | 0.264 |
Table 7: Standard Plot of Naproxen In 6.8 Ph Phosphate Buffer

Figure 3: Calibration curve of naproxen
| Time
(min) |
Cumulative Percentage of Drug Release in pH 6.8 Phosphate Buffer. | |||||
| F1 | F2 | F3 | F4 | F5 | F6 | |
| 10 | 42.8 | 56.6 | 68.6 | 78.6 | 68.4 | 76.8 |
| 20 | 70.6 | 64.8 | 74.8 | 79.8 | 74.8 | 83.6 |
| 30 | 78.4 | 80.6 | 86.4 | 84.8 | 76.6 | 91.8 |
| 40 | 84.6 | 84.8 | 90.6 | 91.4 | 88.4 | 95.8 |
| 50 | 88.8 | 92.8 | 94.8 | 97.8 | 89.6 | 97.4 |
| 60 | 92.6 | 94.7 | 96 | 99.4 | 96.6 | 98.7 |
Table 8: Cumulative Percentage drug Release of F1 – F6in pH 6.8 Phosphate Buffer
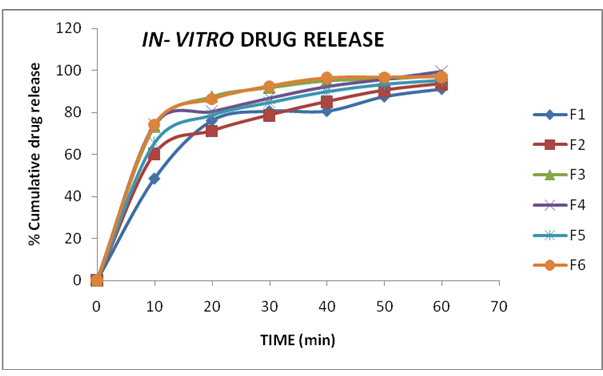
Figure 4: In-vitro drug release of mouth dissolving tablets of Naproxen (F1-F6)
Optimized formula:
The dissolution profile of formulations (F1-F6) indicates a faster release of drug i.e., 90% or more of the drug released from all formulations within 60 mi. However, the maximum release of drug (99.4%) was observed in F4. This formulation also exhibited better performance in the other evaluation parameters. Hence, formulation (F4) is selected as an optimized formula.
| S.No | ingredients | QUANTITY
(mg) |
| 1. | Naproxen | 150 |
| 2. | Microcrystalline cellulose | 10 |
| 3. | Mannitol | 14 |
| 4. | Crospovidine | 60 |
| 5. | Aerosil | 2 |
| 6. | Aspartame | 10 |
| 7. | Magnesium stearate | 4 |
| Total | 250 |
Table 9: Finalized formula
In-vitro dispersion time for naproxen mouth dissolving tablets (F4)
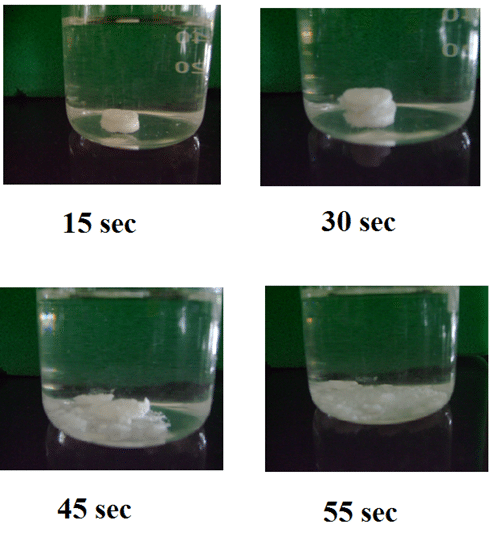
Figure 5: In-vitro dispersion time for naproxen mouth dissolving tablets
Discussion
The demand for orally disintegrating tablets has enormously increased during the last decade. Particularly for geriatric and pediatric patients who have difficulty swallowing conventional tablets and capsules. Fast dissolving or fast disintegrating dosage form is advantageous for such patients. Fast dissolvable or fast disintegrating dosage forms are meant to disintegrate immediately upon contact with the saliva, leading to faster release of the drug in the oral cavity. By administering the fast-disintegrating dosage forms, absorption of the drugs occurs through the buccal mucosa, and it may reduce the first-pass metabolism, leading to better efficacy of the drug.
Abdul Jaleel et al. worked to enhance the dissolution of orally disintegrated tablets of loratadine. Orodispersible tablets of loratadine were prepared using different types and concentrations of superdisintegrants (Ac-Di-Sol, sodium starch glycolate, and crospovidone (CP) (at different particle sizes)) using the direct compression method. As the drug is poorly water soluble, they made solid dispersions of the drug with PVP30 by the solid evaporation method to enhance the solubility. The results revealed that tablets containing CP as a superdisintegrant had a good dissolution profile with the shortest DT.[16]
Singh et al. formulated and evaluated Orodispersible tablets of Norfloxacin using sodium starch glycollate and crospovidone as superdisintegrants, and Camphor is used as the sublimating agent. Cros povidone showed the best in vitro drug release compared to sodium starch glycolate. They used the sublimation technique to formulate the Orodispersible tablets. The results showed that the sublimation method is the best technique for tablets.[17]
Jeevanandham S et al. prepared fast-dissolving tablets of naproxen sodium using camphor as a subliming agent. Orodispersible tablets of naproxen sodium were prepared using the wet granulation technique, using camphor as a subliming agent and sodium starch glycolate together with croscarmellose sodium as superdisintegrants. They optimized the formulation; the results showed the effect of formulation processing variables, especially the subliming agent, on the drug release profile.[18]
R. V. Venky et al. formulated and evaluated Rizatriptan benzoate mouth disintegrating tablets using the superdisintegrants crospovidone, carboxymethyl cellulose calcium, Indion 414, and Indion 234 using the direct compression method. Almost 90% of the drug was released from all formulations within 20 minutes. The formulation was developed with the aim of providing for patients suffering from migraines. It exhibited higher release rates of rizatriptan benzoate.[19]
Vijay Tiwari et al. did research on fast dissolving tablets of Celecoxib using solid dispersion of Celecoxib and sorbitol by holt melt extrusion process, and superdisintegrants. The results showed that the inclusion of the solid dispersion process as a step of preparation of fast dissolving tablets has a synergistic effect on the bioavailability of the final dosage form by its contribution to the improvement in the solubility profile.[20]
Astha Verma et al. studied the progress of evolving technology in the formulation and manufacturing of orally disintegrating tablets prepared using various approaches like freeze drying, compression, molding, and sublimation. They also made a study on patented technology like Wowtab, Durasolv, Orasolv, Flashtab, and Zydis, which have gained importance in the international market.[21]
Rangasamy Manivannan et al. formulated a pharmaceutically stable and robust formulation of Naproxen sodium tablets USP 220mg, comparable with the innovator. In the present study, they reduced the excipients, which reduced the cost of the dosage form. The tablets of Naproxen sodium USP 220mg were successfully prepared by using the wet granulation technique. Invitro studies showed the drug release of 103.5%in 60min which is faster than the innovator product. The results showed it is a better formulation than an innovator.[22]
B. S. Venkateswarlu et al. formulated and evaluated fast-dissolving tablets of carvedilol. The solubility was enhanced by using β-cyclodextrin as a complexing agent. Carvedilol can be successfully complexed with Beta-cyclodextrin to prepare fast-dissolving tablets in the ratio of 1:4.[23]
Ganesh kumar Gudas et al. formulated and evaluated fast dissolving tablets of chlorpromazine HCl tablets were prepared by using sodium starch glycolate, crospovidine, croscarmellose sodium, L-hpc, and pregelatinised starch. it was concluded that the fast-dissolving tablets with proper hardness, disintegrating with enhanced dissolution can be made using selected superdisintegrants.[24]
Hyma P et al. prepared Naproxen Sodium organic dye films (ODFs) using the solvent casting method. The assessment of prepared ODFs involved the consideration of several parameters, including film thickness, folding endurance, disintegration time, surface pH, weight variation, in-vitro dissolution test, content uniformity, and FTIR.[25] The findings indicate that F6 exhibits superior performance as a fast-release formulation, as evidenced by its disintegration time of less than 1 minute and dissolution rate of 103.5% after 30 minutes. The film characteristics of Formulation F6, including weight variation, thickness, pH, and folding endurance, were found to be within the acceptable limits set by the United States Pharmacopoeia (USP). In conclusion, the utilization of oral fast dissolving film (OFDF) presents a novel approach to improve consumer acceptance through its rapid disintegration, convenience of self-administration without the need for water or chewing. The film serves as a highly effective method for delivering medications through the oral route, facilitating rapid dissolution. This method extends to a diverse array of medications, encompassing neuroleptics, cardiovascular drugs, analgesics, anti-asthmatic agents, antihistamines, and drugs targeting erectile dysfunction. Oromucosal medication delivery is considered advantageous from the patient’s perspective due to its feasibility of administration without the requirement of swallowing, thereby enhancing patient safety. The concept of a rapid-dissolving dosage form has become increasingly popular as a novel method of drug delivery. This approach aims to achieve optimal therapeutic efficacy, enhanced bioavailability, and maximum stability by reducing the frequency of dosage administration. Furthermore, this will overcome the process of first-pass metabolism of drugs. This approach facilitates expedited absorption of medication from the pre-gastric region, potentially leading to an accelerated initiation of therapeutic effects.
Conclusion
In the present work, an attempt was made to develop mouth-dissolving tablets of naproxen by the direct compression method and by using crospovidone, croscarmellose sodium, and sodium starch glycolate as superdisintegrants. In the preformulation studies, it has been proved that there is no interaction between the drug and the excipients. The blends of varying superdisintegrants were formulated into 6 formulations ranging from F1 to F6, and the blends were evaluated for the pre- and post-comparison parameters, and in vitro drug release was also studied. All the pre-compression parameters, angle of repose, Cars index, Hausner’s ratio, tapped and bulk density, are within the limits. The results showed that the formulations containing the crospovidone have good flow properties and good compatibility when compared with the other formulations. The post-compression parameters include the Weight variation, Hardness, friability, Thickness, wetting time, in vitro disintegration, in vitro dispersion time, and the water absorption ratio. The results show maximum for the formulation (F4) that contains crospovidone as a superdisintegrant. The In vitro drug release of formulation F4 had shown that maximum drug release 99.4 ± 0.54 when compared with the other formulations. So, F4 was chosen as the best formulation, which contains crospovidone as a superdisintegrant. The naproxen 250 mg mouth dissolving tablet was prepared by using the finalized formula and optimized manufacturing process, which showed good results in the formulation of a stable tablet dosage form.
References
- Brahmankar DM, Jaiswal SB. Biopharmaceutics & Pharmaceutics – A Treatise. 3rd ed. Vallabh Prakashan; 2015. Biopharmaceutics & Pharmaceutics – A Treatise
- Ansel HC, Popvich NG, Allen LV. Pharmaceutical Dosage Forms and Drug Delivery Systems. 10th ed. Lippincott Williams & Wilkins; 2017. Pharmaceutical Dosage Forms and Drug Delivery Systems
- Biradar S, Bhagavati S, Kuppasad I. Fast dissolving drug delivery systems: A brief overview. Internet J Pharmacol. 2005;4(2). Fast dissolving drug delivery systems
- Kuccherkar BS, Badhan AC, Mahajan HS. Mouth dissolving tablets: A novel drug delivery system. Pharma Times. 2003;25:43-47. Mouth dissolving tablets: A novel drug delivery system
- Kaushik D, Dureja H, Saini TR. Mouth dissolving tablets: A review. Indian Drugs. 2004;41(4):187-193. Mouth dissolving tablets: A review
- Roser BJ, Blair J. Rapidly soluble oral dosage form, method of making same and composition. U.S. Patent No. 5,762,961; 1998. Rapidly soluble oral dosage form, method of making same and composition
- Lachmann L, Lieberman HA, Kanig JL. The Theory and Practice of Industrial Pharmacy. 3rd ed. Varghese Publishing House; 1998. The Theory and Practice of Industrial Pharmacy
- Indian Pharmacopoeia. Vol. II. Controller of Publications, Ministry of Health; 1996. Controller of Publications, Ministry of Health
- Gudas G, Manasa B, Rajesham VV, Kiran KS, Prasanna JK. Formulation and evaluation of fast dissolving tablets of chlorpromazine HCl. J Pharm Sci Tech. 2010;2(1):99-102. Formulation and evaluation of fast dissolving tablets of chlorpromazine HCl
- U.S. Pharmacopoeia. Available from: U.S. Pharmacopeia (USP). 2024. U.S. Pharmacopeia
- Kumar D, Vuyyuru T, Kollipara D. Fast dissolving tablets – An overview. Int J Res Pharm Sci. 2012;3(2):348-355. Fast dissolving tablets – An overview
- Ghourichay MP, Kiaie SH, Nokhodchi A, Javadzadeh Y. Formulation and quality control of orally disintegrating tablets (ODTs): Recent advances and perspectives. Biomed Res Int. 2021;6618934. doi:10.1155/2021/6618934 PubMed | Crossref | Google Scholar
- Roy D, De A, Biswas S, Pramanick N, Das A, Mondal S. An overview on mouth dissolving tablets. J Huazhong Univ Sci Technol (Nat Sci Ed). 2022;50(4):1-30. An overview on mouth dissolving tablets
- Raghavendra Rao NG, Thube K, Sumanji Bala. Formulation and evaluation of fast dissolving tablets of metoprolol tartrate using super disintegrants. Int J Pharm Clin Res. 2010;2(1):40-45. Formulation and evaluation of fast dissolving tablets of metoprolol tartrate using super disintegrants
- Gosai AR, Patil SB, Sawanth KK. Formulation and evaluation of orodispersible tablets of ondansetron hydrochloride by direct compression using super disintegrants. Int J Pharm Sci Nanotech. 2008;1(1):106-111. Formulation and evaluation of Oro-dispersible tablets of ondansetron hydrochloride by direct compression using super disintegrants
- Jaleel OW, Abdul Rasool AA, Ghareeb M. Preparation and characterization of orally disintegrating loratadine tablets from PVP solid dispersions. Int J Pharm Sci. 2010;2(3):759-770. Preparation and characterization of orally disintegrating loratadine tablets from PVP solid dispersions
- Singh VK, Maurya JK, Mishra P, Singh PK, Mishra V. Formulation and evaluation of mouth dissolving tablet of norfloxacin with piperine and their antibacterial activity. Am J Pharm Tech Res. 2013;3(5). Formulation and evaluation of mouth dissolving tablet of norfloxacin with piperine and their antibacterial activity
- Jeevanandham S, Dhachinamoorthi D, Chandra Sekhar KB, et al. Formulation and evaluation of naproxen sodium orodispersible tablets using a sublimation technique. Asian J Pharmaceutics. 2010;4(1):48-51. doi:10.4103/0973-8398.63985 Crossref | Google Scholar
- Venky RV, Desouza C, Lourenco CF. Formulation and evaluation of rizatriptan benzoate mouth dissolving tablets. Indian J Pharm Sci. 2010;72(1):79-85. doi:10.4103/0250-474X.62253 PubMed | Crossref | Google Scholar
- Tiwari V, Kinikar D, Pillai K, Gokulan PD. Preparation and evaluation of fast dissolving tablets of celecoxib. J Current Pharm Res. 2010;4:4-11. Preparation and evaluation of fast dissolving tablets of celecoxib
- Verma A, Biswas D, Madhukar V. Orally disintegrating tablets: Boon for market and franchises: A review. 2010. Semantic Scholar. Orally disintegrating tablets: Boon for market and franchises: A review
- Rangasamy M, Manivannan K, Ganesan K, et al. Formulation development and evaluation of naproxen sodium tablets USP. Int J Drug Dev Res. 2010;2(1):47-53. Formulation development and evaluation of naproxen sodium tablets USP
- Venkateswarlu BS, Chandrika R, Talele AJ, et al. Formulation development and evaluation of fast dissolving tablets of carvedilol. J Chem Pharm Res. 2010;2(1):196-210. Formulation development and evaluation of fast dissolving tablets of carvedilol
- Gudas G, Manasa B, Rajesham VV, Kiran KS, Prasanna JK. Formulation and evaluation of fast dissolving tablets of chlorpromazine HCl. J Pharm Sci Tech. 2010;2(1):99-102. Formulation and evaluation of fast dissolving tablets of chlorpromazine HCl
- Hyma P, Fatima S. Formulation and evaluation of oral fast dissolving films of naproxen sodium. Int J Current Pharm Res. 2022;14(2):48-53. doi:10.22159/ijcpr.2022v14i2.1953 Crossref
Acknowledgments
Not applicable
Funding
Not applicable
Author Information
Corresponding Author:
Raziya Begum Sheikh
Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Email: raziya.pharma@gmail.com
Co-Authors:
Samatha Ampeti
Department of Pharmacology
Kakatiya University, University College of Pharmaceutical Sciences, Warangal, TS, India
Email: ampetisamatha9@gmail.com
Sonam Shashikala B V
Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Email: venkateshsonams@gmail.com
Mansi Srivastava
Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Email: srivastavamansi811@gmail.com
Shubham Ravindra Sali
Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Email: shubhamsali42@gmail.com
Author Contribution
The author contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles and was involved in the writing – original draft preparation and writing – review & editing to refine the manuscript.
Informed Consent
Not applicable
Conflict of Interest Statement
Not applicable
Guarantor
Not applicable
DOI
Cite this Article
Raziya BS, Samatha A, Sonam SBV, Mansi S, Shubham RS. Development and Assessment of Naproxen Mouth Dissolving Tablets. medtigo J Pharmacol. 2024;1(1):e3061112. doi:10.63096/medtigo3061112 Crossref









