Author Affiliations
Abstract
Background: Diabetic macular edema (DME) can significantly hinder the life of many diabetic patients. Research has shown that intravitreal injections and laser effectively target this serious complication. This study aims to compare the effectiveness and safety of ranibizumab and triamcinolone when combined with prompt laser in treating DME.
Methods: We systematically gathered research papers from the major databases, including PubMed, Cochrane Library, Scopus, Elsevier, EBSCO, and Sage. Following the appropriate guidelines tailored towards contrasting the effectiveness and safety between Ranibizumab and Triamcinolone in patients with DME.
Results: This meta-analysis found that ranibizumab is more effective in targeting DME in terms of optical coherence tomography (OCT) readings and retinal structures, whereas triamcinolone was associated with better disease control and fewer systemic side effects.
Conclusion: These results demonstrate the need for a patient-centred treatment plan that considers the efficacy and safety of each drug combined with the individual risk factors of each patient with DME.
Keywords
Diabetic macular edema, Diabetes mellitus, Ranibizumab, Triamcinolone, Laser treatment.
Introduction
Diabetes mellitus (DM) is the most prevalent endocrine disorder globally. Due to effective treatment options, people started to live longer, which increases the risk of long-term complications such as macrovascular issues like coronary artery disease and cerebrovascular disease, as well as microvascular complications including neuropathy, nephropathy, and retinopathy. Diabetic retinopathy is the leading cause of blindness among individuals aged 15-65 in the developed world. Various eye complications, including DME, which is considered a serious manifestation of advanced diabetic retinopathy, can lead to significant visual impairment. Management strategies range from lifestyle changes, hypoglycaemic medications, and insulin for diabetes control, to more advanced treatments like intravitreal injections and laser therapy.[1-3]
Intra-vitreal injections, particularly, have revolutionized the management of DME. In this research, we aimed to discuss various forms of treatment, including focusing on comparing intravitreal injections such as anti-VEGF (vascular endothelial growth factor) agents (bevacizumab, ranibizumab, aflibercept) and steroids (dexamethasone, triamcinolone), used alone or in combination with laser therapy. However, there is limited secondary research or meta-analysis comparing the efficacy and safety of ranibizumab versus triamcinolone with adjunctive laser therapy. To address this gap, we conducted a systematic review and meta-analysis to evaluate the safety and effectiveness of ranibizumab and triamcinolone with laser therapy in managing DME.
Methodology
Search strategy following guidelines specified in the preferred reporting items for systematic reviews and meta-analyses (PRISMA), we systematically searched electronic databases, including PubMed, Scopus, Cochrane Library, Elsevier journals, spanning from each database’s inception to October 2, 2024. Our search strategy involved a combination of relevant keywords and standardized index terms tailored to the comparison between the effectiveness of ranibizumab and triamcinolone in patients with DME.
Selection criteria and quality assessment
Type of studies: This review and meta-analysis will look at studies that followed patients over time or looked back at records. These studies need to include ten or more people with DME. The research compared what happened to patients who took ranibizumab versus those who took triamcinolone. All patients in the studies should have DME.
Types of intervention: Patients will be treated with triamcinolone along with prompt laser therapy. Both treatments are aimed at reducing retinal edema and improving visual acuity in patients suffering from DME. The ranibizumab group will receive a dose of 0.5 mg, a widely used anti-VEGF (vascular endothelial growth factor) agent known for its efficacy in managing retinal conditions. Conversely, the triamcinolone group will receive a 4 mg intravitreal injection of the corticosteroid, which is effective in reducing inflammation and fluid accumulation in the retina. Each treatment will be compared to a placebo to evaluate the true efficacy of the interventions. This design not only aims to assess the individual effectiveness of ranibizumab and triamcinolone but also seeks to provide insight into the potential additive effects of combining these therapies with prompt laser treatment.
Types of outcome measures: Eligible studies must focus on clinically relevant outcomes regarding the efficacy and safety of ranibizumab and triamcinolone. These results can include complications on various systems such as cardiac failure, congestion, anemia, hypothyroidism, increases in IOP>30, and vitreous hemorrhage.
Exclusion criteria: In our study, the intervention involves administering two distinct treatment regimens for patients with DME.
One group will receive ranibizumab combined with prompt laser therapy, while the other group will receive inadequate reporting of outcomes, such as failing to specify the exact number of events and the total patient-years for comparisons between the drug approaches, will be excluded.
Studies will be excluded if they: do not involve human subjects; are not published in English; have a sample size smaller than ten in either ranibizumab or triamcinolone; present overlapping data from the same institution(s); or have a median follow-up duration of less than 3 months.
Furthermore, studies that do not report outcomes specifically for the effects of ranibizumab and triamcinolone will be excluded. Reviews, case reports, conference abstracts, letters to the editor, and editorials will also be excluded.
Quality assessment: Before conducting the statistical analysis, we assessed the risk of bias (ROB) and the quality of the included studies. For the two eligible RCTs, we used the revised RoB-2 Cochrane tool and conducted the assessment using Cochrane Review Manager Web.
Affect duration of diabetes on DME: The duration of diabetes significantly affects the likelihood and severity of DME in patients. Longer durations of diabetes are associated with an increased risk of developing DME, as well as more severe retinal damage.
Stages of diabetes:
- Early stages of diabetes (0–10 years): In patients with shorter durations of diabetes, DME may occur but tends to be less severe. Diabetic retinopathy can still be mild, and microvascular changes in the retina are usually less extensive.
- 10–20 years of diabetes: As diabetes progresses over time, microvascular complications like DME become more common. The cumulative damage to the retinal capillaries leads to increased leakage of fluids into the macula, resulting in DME. Patients in this range are significantly more likely to develop vision-threatening DME.
- >20 years of diabetes: Patients who have had diabetes for more than 20 years are at a very high risk of developing not just DME but also advanced diabetic retinopathy, including proliferative diabetic retinopathy (PDR). The longer exposure to high blood sugar levels can lead to extensive capillary damage, leading to severe macular edema and vision loss.
In studies examining the duration of diabetes in patients with DME, the range generally falls between 14 to 18 years. Patients[1] receiving ranibizumab had a diabetes duration of 14 years, while those receiving triamcinolone had a slightly longer duration of 14.3 years. It reported similar findings, with patients in the ranibizumab prompt treatment group having a diabetes duration of 18 years, and those in the deferred group having a duration of 17 years; triamcinolone patients across both prompt and deferred groups had a duration of 17 years.[2] It observed a diabetes duration of 15 years for both ranibizumab and triamcinolone groups.[3]
In the study, the duration was 16 years for patients receiving ranibizumab and 18 years for those receiving triamcinolone.[4] These findings suggest that diabetes duration tends to be similar across treatment groups in patients with DME.
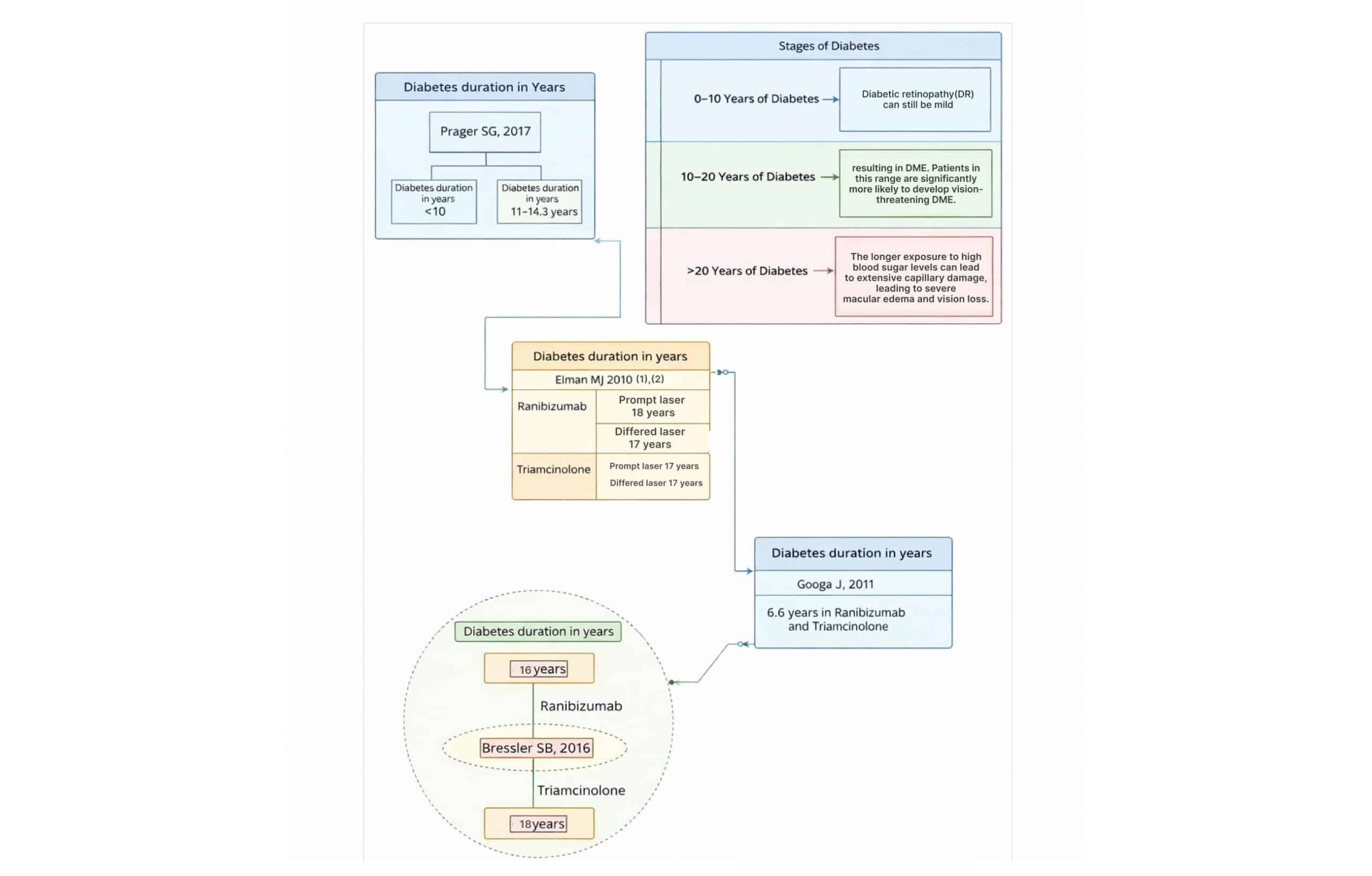
Figure 1: The stages of diabetes and diabetes duration in years for studies
The longer a patient has diabetes, the higher the risk and severity of DME. The risk is particularly heightened after 10 to 20 years of diabetes, and those with more than 20 years are at the highest risk. Early intervention and tight control of blood sugar levels can mitigate the progression of DME in long-term diabetes patients.
Results
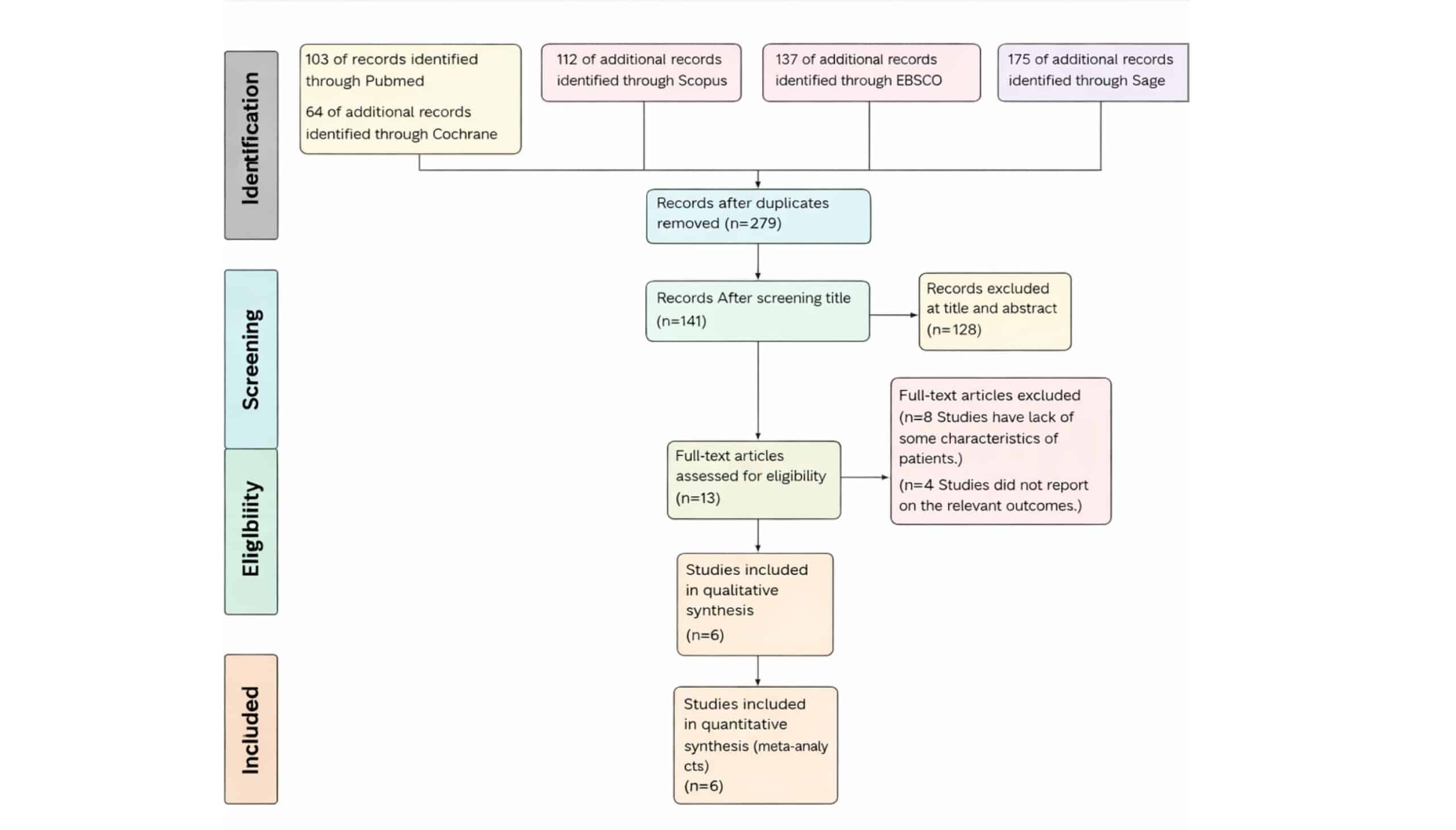
Figure 2: The flowsheet of search results according to PRISMA guidelines
PRISMA flow diagram provides a detailed account of the selection process for the systematic review and meta-analysis on the use of ranibizumab compared to triamcinolone in the management of patients with diabetic macular edema. The initial search yielded a total of 591 records from various databases, 103 records from PubMed, 112 records from Scopus, sixty-four records from Cochrane, and 312 additional records identified through other sources (Sage and Ebsco). After removing duplicates, 273 records remained for further evaluation. The screening process began with these 273 records, from which 128 were excluded for focusing on other interventions or being irrelevant to the research question (title and abstract). This exclusion left 128 records for title screening. Following this, 7 more records were excluded for various reasons: 4 due to re-excision rate not being among the outcomes,3 due to a lack of some characteristics of patients. 13 full-text articles were assessed for eligibility, and 6 studies met the inclusion criteria. These 6 studies were subsequently included in both the systematic review and the quantitative synthesis (meta-analysis). This thorough selection process underscores the rigorous methodology employed to ensure the relevance and quality of the studies included in the review, thereby strengthening the reliability of the review’s findings on ranibizumab compared to triamcinolone in the management of patients with diabetic macular edema.

Table 1: Summary of the six studies compared (ranibizumab vs triamcinolone) in patients with DME
Table 1 presents data from six randomized controlled trials conducted across various countries, focusing on patients with DME. In addition, two key studies were highlighted for their focus on patients who had difficulty controlling their DME or were lost to follow-up.[1-4]
One of the studies involved 25 patients divided into two groups: one group received 0.5 mg of ranibizumab, and the other group was treated with 8 mg of triamcinolone. This study focused on key outcomes such as best-corrected visual acuity (BCVA) and central retinal thickness (CRT). Additionally, the study provided calculated values for the thickness of the outer and inner retinal layers, offering deeper insight into retinal changes.[1]
Another study, on the other hand, involved 558 eyes from 450 participants who completed the 5-year extension study, while 270 eyes from 223 patients did not complete the study. This research focused on long-term follow-up and outcomes, providing valuable insights into the sustained effects of treatment.[5]
One of the authors conducted another pivotal trial involving 691 patients with DME (854 eyes). This study compared several treatment groups: patients received either sham treatment, prompt laser, or ranibizumab with either prompt or deferred laser, versus triamcinolone with prompt laser. In the first year of the study, 65 eyes could not complete follow-up, while 270 eyes dropped out in the second year.[2]
In a subsequent study, the focus was on 642 eyes that completed the study out of the original 854 participants. Patients in this trial were divided into those receiving 0.5 mg of ranibizumab with either prompt or deferred laser, compared to those treated with 8 mg of triamcinolone with prompt laser over a 2-year follow-up period.[3]
The fifth study included in the analysis involved 345 eyes and investigated treatments for DME from a 14-week to 56-week visit. This study also reported adverse events observed during follow-up, contributing to the safety profile of the treatments studied.[4]
Lastly, conducted a study involving 792 eyes. In this trial, 9% of patients receiving ranibizumab with prompt laser without PDR did not complete the study. Among patients receiving triamcinolone with prompt laser without PDR, 4% did not complete the study.[5]
Additionally, among patients with PDR, 5% of those treated with ranibizumab and prompt laser, and 6% of those treated with triamcinolone and prompt laser, were lost to follow-up.
Diagnostic findings: The diagnostic images provide a comprehensive overview of retinal abnormalities observed in the patient.
Fundus photography is an imaging technique we can use to capture detailed images of the interior surface of the eye, particularly the retina, optic disc, macula, and posterior pole of the eye. It involves using a specialized camera with a low-power microscope attached, which illuminates the eye with a flash of light, and provides a clear view of blood vessels and nerve fibers.
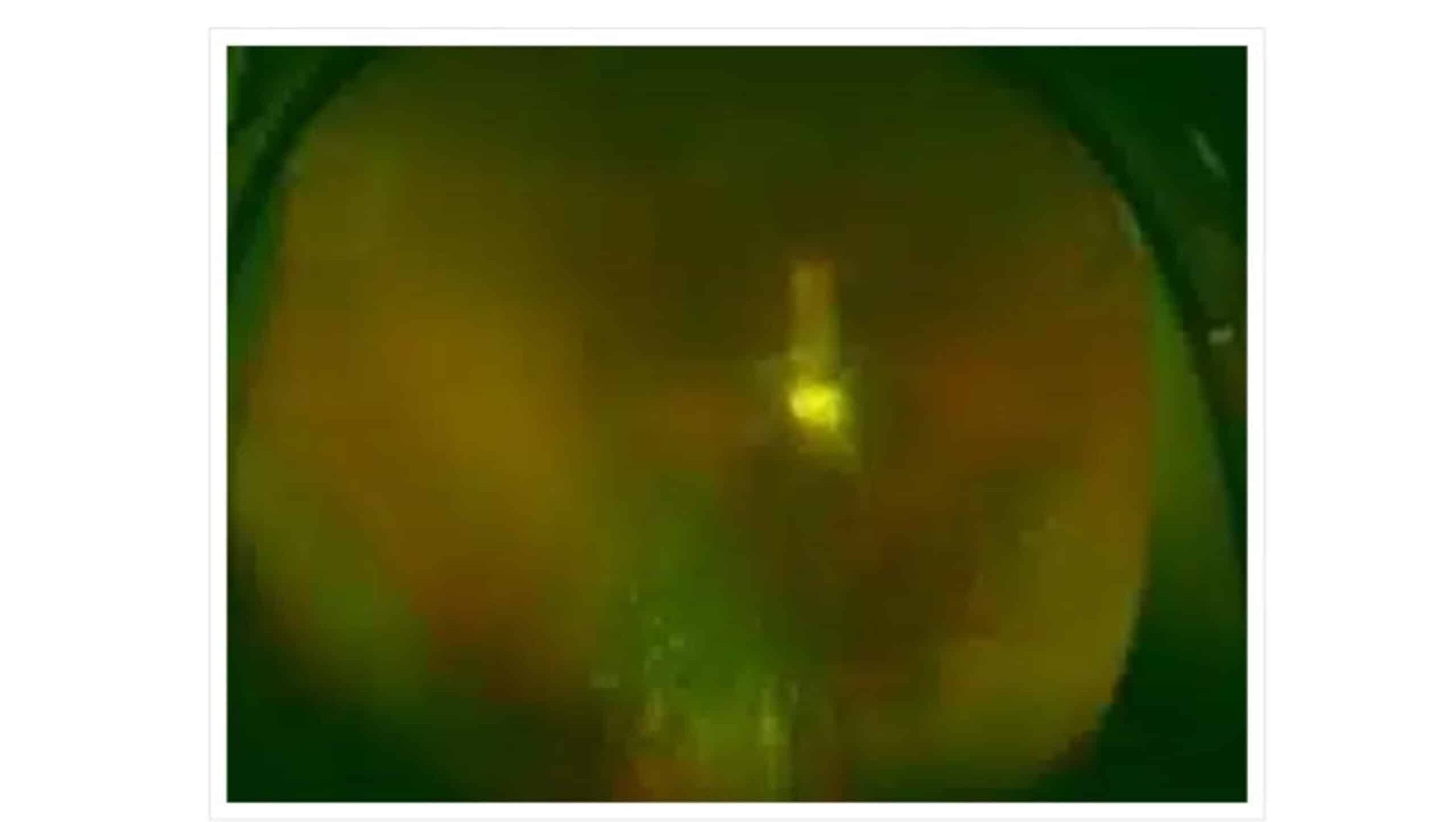
Figure 3: Fundus photography findings: Off white swelling lesions below the optic disc
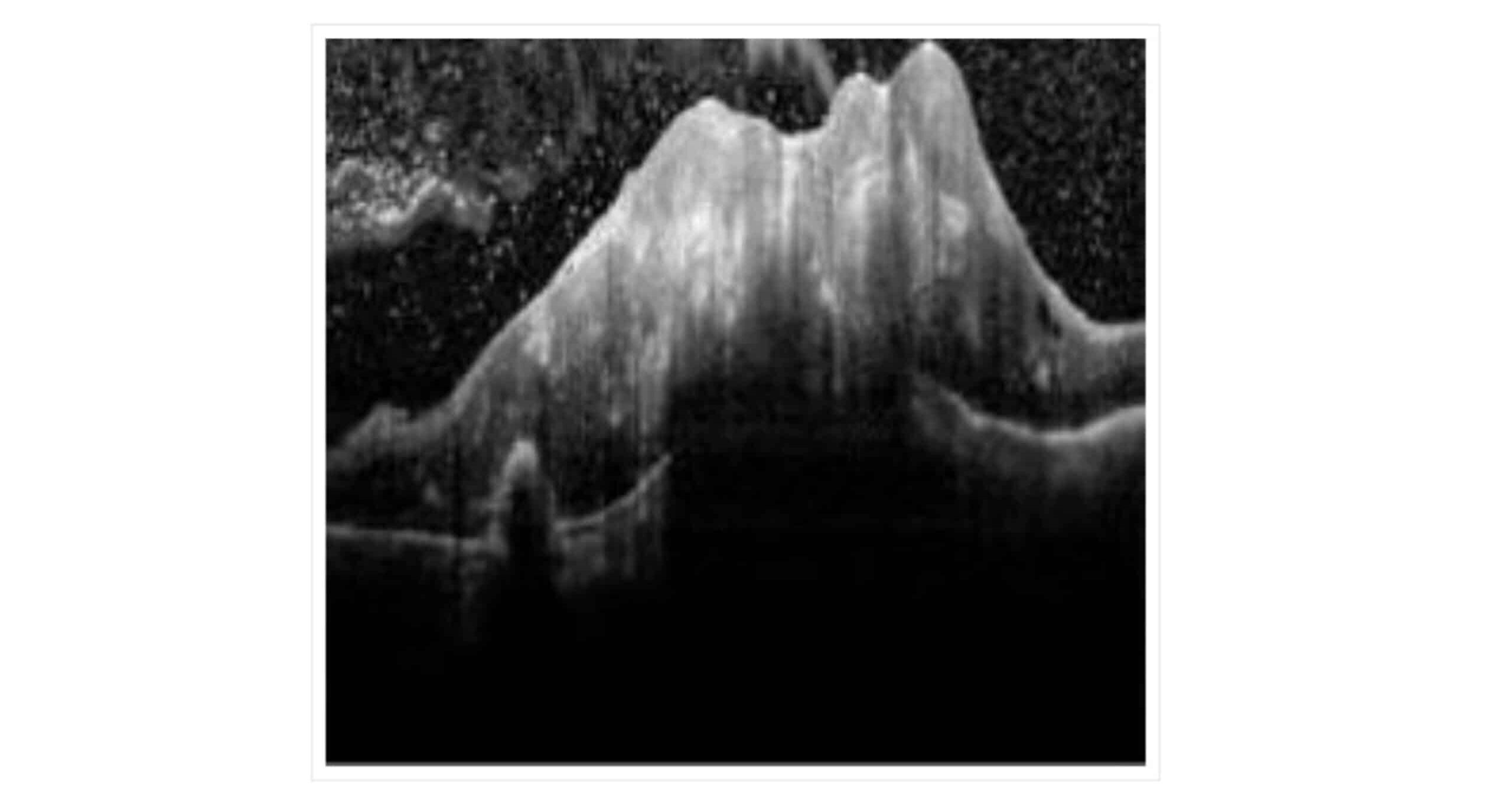
Figure 4: OCT findings: Retinal thickening at the focal site with structural disturbances in the retina featuring areas of high and low reflectance
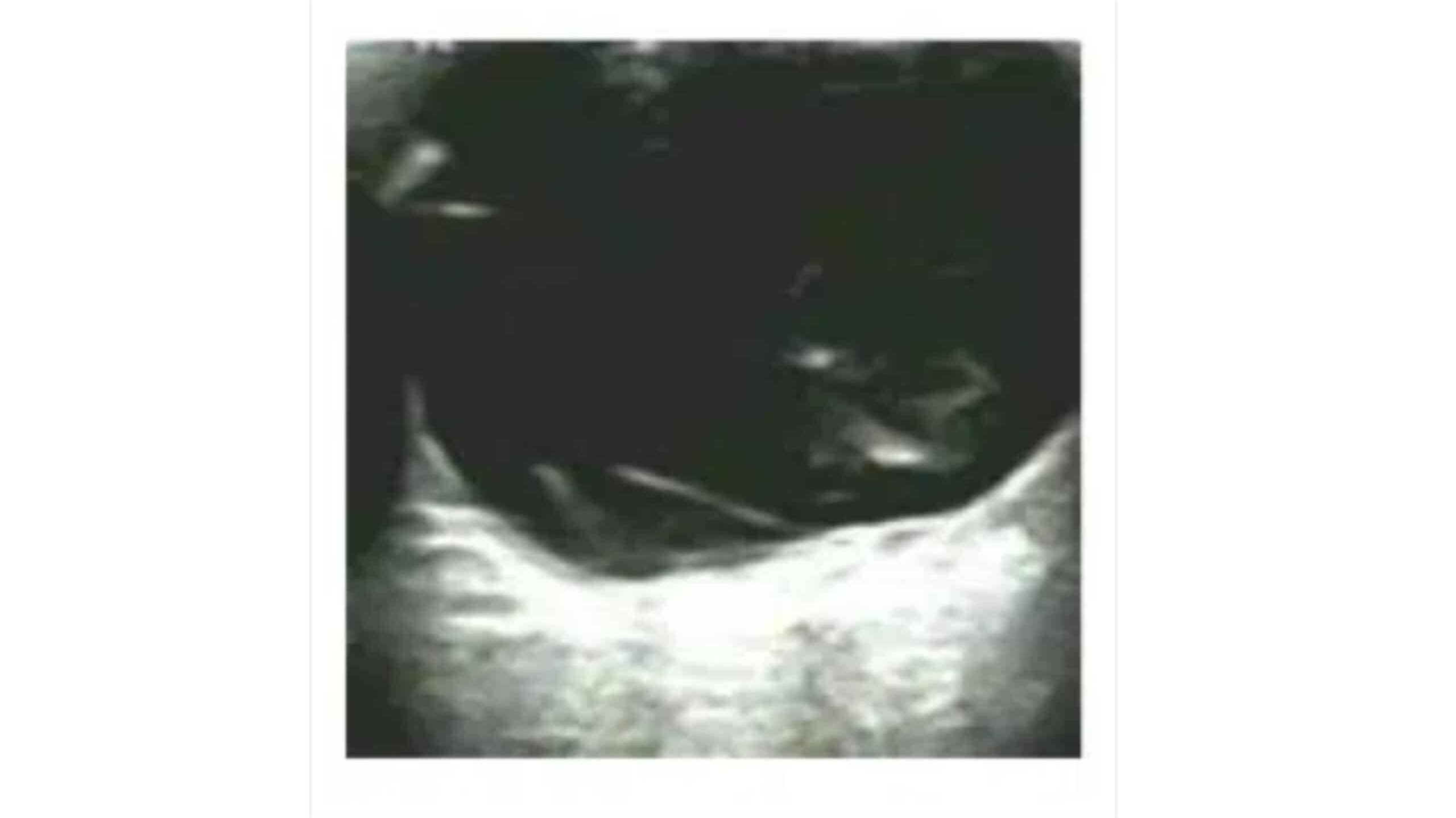
Figure 5: Color ultrasound. Thickening of the retina at the lesion
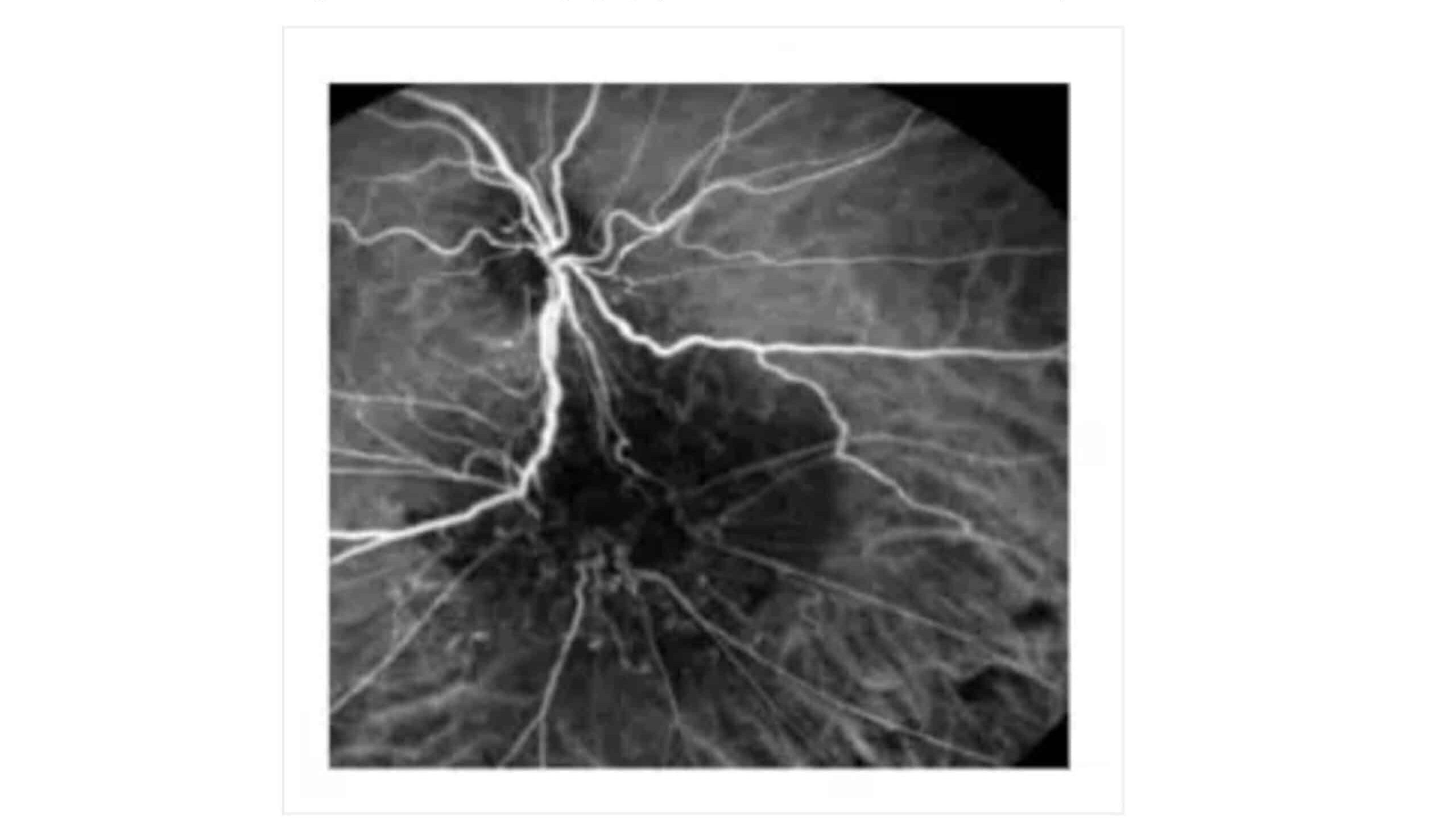
Figure 6: Fluorescein angiography. Twisted blood vessels below the optic
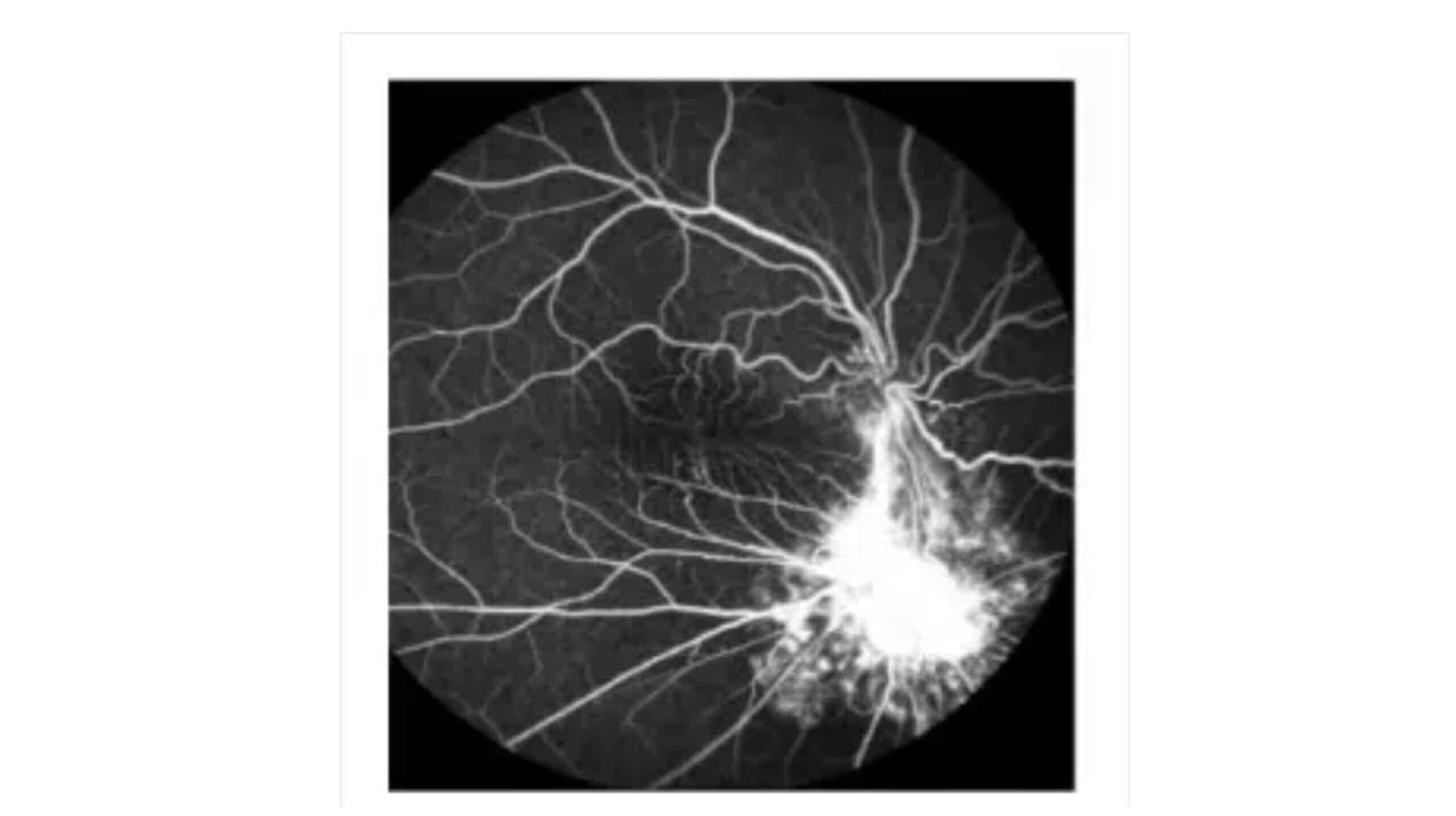
Figure 7: Indocyanine green choroidal angiography, Persistent low fluorescence below the optic disc
Fundus photography (A) reveals off-white swelling lesions below the optic disc, along with a horseshoe-like tear in the temporal peripheral retina, suggesting possible retinal detachment or associated pathology (Figure 3). Optical coherence tomography (OCT) demonstrates significant retinal thickening at the focal site, with visible structural disturbances characterized by areas of high and low reflectance, indicating possible oedema and damage to the retinal layers (Figure 4). Color ultrasound (C) supports these findings, showing retinal thickening at the lesion site, accompanied by stretching and uplifting of the proliferative membrane (Figure 5). A sound shadow is also noted behind the moderate-strength patchy echo, which may indicate fibrosis or scar tissue. Fluorescein angiography highlights twisted blood vessels below the optic disc, with notable fluorescein leakage, suggesting abnormal vasculature and potential vascular leakage (figure 6), while indocyanine green choroidal angiography (figure 7) reveals persistent low.
Finally, indocyanine green choroidal angiography (E) reveals persistent low fluorescence below the optic disc, pointing to potential choroidal circulation issues or hypoperfusion in the affected area (Figure 7).
In conclusion, the combination of imaging modalities, fundus photography, OCT, color ultrasound, fluorescein angiography, and indocyanine green choroidal angiography provides a comprehensive and corroborative diagnosis of significant retinal pathology. The findings, including swelling lesions, retinal thickening, proliferative membrane changes, vascular leakage, and low choroidal fluorescence, suggest a complex condition, potentially involving retinal detachment, proliferative retinopathy, or choroidal vascular abnormalities. These diagnostic insights highlight the importance of a multidisciplinary imaging approach in accurately assessing and guiding the management of retinal diseases, ensuring timely and appropriate intervention to preserve visual function.
| Study ID | Treatment | Age | Duration | Gender | Diabetes duration in years | BCVA After 12 months | |
| Female | Male | ||||||
| Prager 2017 | Ranibizumab | 61.7 ± 8.5 | 1 year | 20% | 80% | 14 years | 0.18 ± 0.20 (20/30) |
| Triamcinolone | 59.5 ± 18.6 | 33.3% | 66.7% | 14.3 years | 0.36 ± 0.19 (20/50) | ||
| Bressler 2016 | Ranibizumab | 65 | 1 year | 16 years | |||
| Triamcinolone | 62 | 18 years | |||||
| Elman 2010
(1) |
Ranibizumab
+Prompt laser |
62 (56, 70) | 1 year | 45% | 55% | 18 years | +9 ± 11 |
| Ranibizumab
+deferred laser |
64 (58, 70) | 41% | 59% | 17 years | +9 ± 12 | ||
| Triamcinolone
+ Prompt laser |
62 (55, 70) | 46% | 54% | 17 years | +4 ± 13 | ||
| Elman 2010
(2) |
Ranibizumab
+Prompt laser |
62 (56, 70) | 2 years | 45% | 55% | 18 years | +7 ± 13 |
| Ranibizumab
+deferred laser |
64 (58, 70) | 41% | 59% | 17 years | +9 ± 14 | ||
| Triamcinolone
+ Prompt laser |
62 (55, 70) | 46% | 54% | 17 years | +2 ± 19 | ||
| Googe 2011 | Ranibizumab
+Prompt laser |
57 (48, 64) | 14 -56
Weeks |
48% | 52% | 15 years | |
| Triamcinolone
+ Prompt laser |
58 (49, 64) | 44% | 56% | 15 years | |||
| Bressler 2013 | Ranibizumab
+Prompt Laser |
64 (58, 71) | 3 years | 45% | 55% | 16 years | |
| Ranibizumab
+Deferred Laser |
64 (58, 70) | 38% | 62% | 15 years | |||
| Triamcinolone
+Prompt Laser |
64 (57, 71) | 44% | 56% | 16 years |
Table 2: Summary of patients’ characteristics for six studies
Table 2 compares the patient characteristics across six studies focusing on the treatment of DME with ranibizumab and triamcinolone. In one of the studies, 25 patients were included in both the ranibizumab and triamcinolone groups. In the ranibizumab group, 80% were male and 20% female, while the triamcinolone group comprised 66.7% male and 33.3% female patients. The average age in the ranibizumab group was 61.7 ± 8.5 years, compared to 59.5 ± 18.6 years in the triamcinolone group.[1]
The study reported similar demographic data. In the ranibizumab group, males made up 55% and 62% of the patients with prompt and deferred laser, respectively, while females constituted 45% and 38% in these subgroups. In the triamcinolone group, males accounted for 44% and females 56%.[6]
The age range in both groups was comparable, with the ranibizumab group having a median age of 64 (58,71) and the triamcinolone group showing a median age of 64 (57,71).
The two studies provided consistent data for both age and gender distributions. In both studies, the ranibizumab + prompt Laser group had an age range of 62 (56,70), while the ranibizumab + deferred Laser group had a range of 64 (58,70). The triamcinolone + prompt laser group had a similar range of 62 (55,70). In terms of gender, 54% to 59% of the patients were male, while 41% to 46% were female.[1,2]
Another study also focused on patients undergoing prompt laser treatment, either with ranibizumab or triamcinolone. The ranibizumab group had a median age of 57 (48,64) and the triamcinolone group had a median age of 58 (49,64). In both groups, males were more prevalent, with 52% and 56% in the ranibizumab and triamcinolone groups, respectively, while females made up 48% and 44%.[3]
In terms of visual outcomes, best corrected visual acuity (BCVA) after 12 months in the Prager SG et al.[1] the study was 0.18 ± 0.20 in the ranibizumab group compared to 0.36 ± 0.19 in the triamcinolone group. In the Elman MJ (2010) (1) study, BCVA improvement in the ranibizumab + prompt Laser group was +9 ± 11, and for the ranibizumab + deferred Laser group, it was +9 ± 12, while the triamcinolone + prompt laser group showed an improvement of +4 ± 13. Similarly, Elman MJ et al.[2] study, BCVA was +7 ± 13 in the ranibizumab + prompt Laser group, +9 ± 14 in the ranibizumab + deferred Laser group, and +2 ± 19 in the triamcinolone + prompt Laser group.
Classification of severity of diabetes retinopathy: Early treatment diabetic retinopathy study (ETDRS) is a system used to classify the severity of diabetic retinopathy, whether it is non-proliferative (NPDR) or proliferative (PDR). It is based on checking physical findings like microaneurysms, hemorrhages, and the growth of new blood vessels in the retina. These changes help in identifying the stage of the disease and deciding the treatment. The data I reviewed compares two studies that evaluate the effects of ranibizumab and triamcinolone at different ETDRS levels.
The goal is to see how well each treatment works as the disease progresses.
For NPDR: At Levels 35 and 43, which are early NPDR stages with mild to moderate findings like microaneurysms. In the first group, 5 patients received ranibizumab and 6 received triamcinolone. The numbers are remarkably close, so there’s no major difference between the treatments at this point, but in the third group, 103 patients received ranibizumab, and 95 patients received triamcinolone, which shows Triamcinolone is still slightly more common in this early stage. At level 47, which is moderately severe NPDR, where we see more hemorrhages in the first group, ranibizumab was used in 15 patients and triamcinolone in 10, while the third study jumps to 103 patients with ranibizumab and ninety-five patients with triamcinolone. Again, there’s a slight preference for ranibizumab, but triamcinolone is still widely used. At level 53, which represents severe NPDR with more damage, In the first group, both treatments were used equally, 6 patients for ranibizumab and 5 for triamcinolone. The third study included 16 patients for ranibizumab and 15 for triamcinolone; both treatments seem equally effective at this stage.
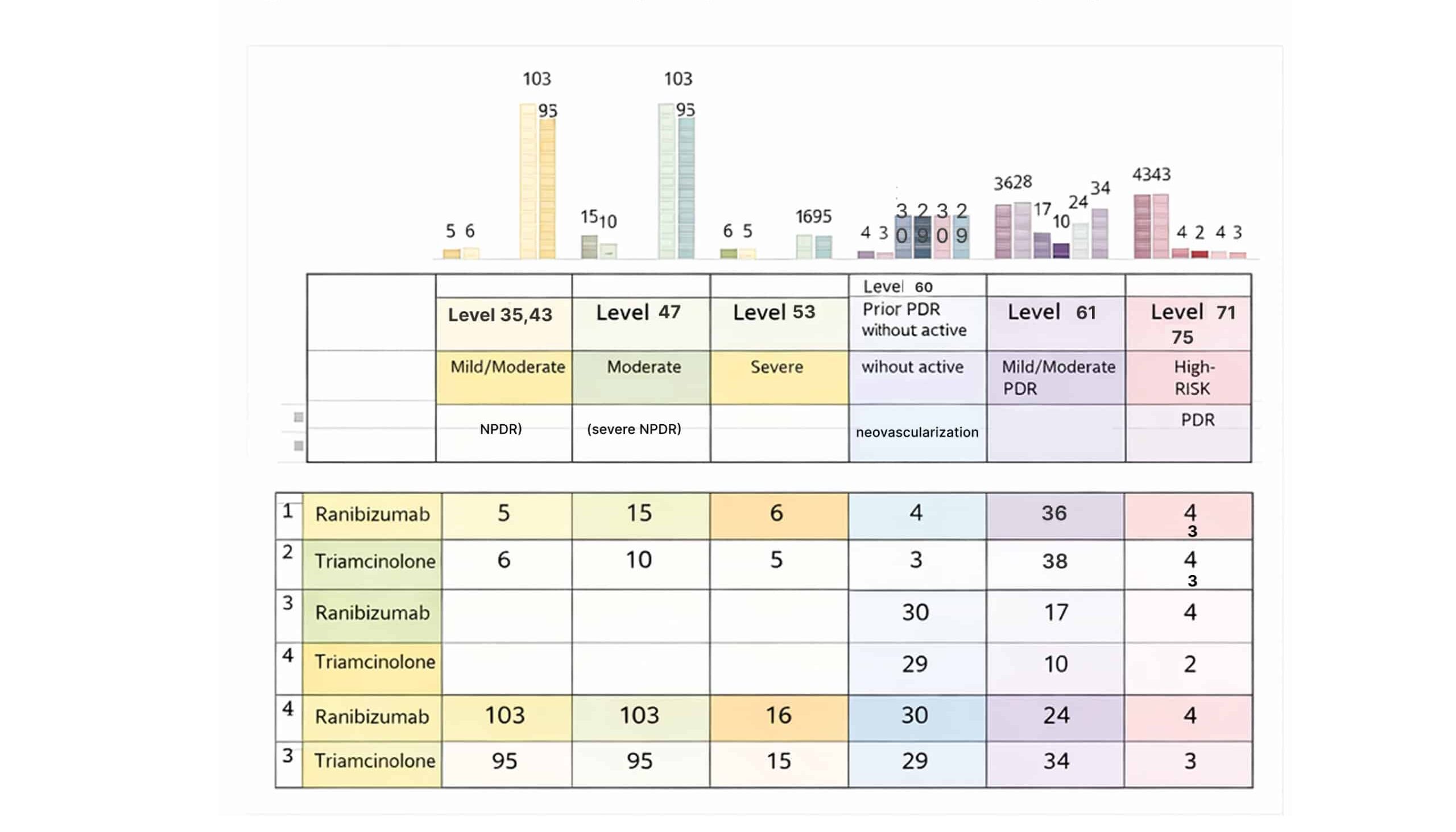
Figure 8: Classification of severity in a patient with diabetes retinopathy
For PDR: At level 60, this level is for patients with PDR who had platelet-rich plasma (PRP) before but don’t have any new blood vessels right now. In the second group, 17 patients got ranibizumab, and 10 patients got triamcinolone. Doctors like ranibizumab more at this stage because it helps prevent new blood vessels from developing after laser treatment. In the first group, there were 4 for ranibizumab and 3 for triamcinolone. So, there is a little preference for ranibizumab here. Also, In the third group, 30 patients for ranibizumab and 29 for triamcinolone, which again showing a general preference for ranibizumab across all groups. At level 61, this level for patients mild to moderate PDR where some new blood vessels are starting to show up.
In the first group, 36 patients got ranibizumab and 38 got triamcinolone. Showing an almost equal use. However, in the second group, 8 patients received triamcinolone, while only 1 patient received ranibizumab. In the third study, 24 patients got ranibizumab and 34 got triamcinolone, demonstrating a slightly stronger preference for triamcinolone at this stage. At levels 71 and 75, these levels are for high-risk PDR where new blood vessels are more of a problem. In the first group, both treatments were used equally, with 43 patients each for ranibizumab and triamcinolone. So, there is an equal preference. In the second group, 4 patients got ranibizumab and 2 got triamcinolone. Even with smaller numbers, it seems that ranibizumab is still preferred a bit more. In the third study, the numbers remain like the first, with 4 patients on ranibizumab and 3 on triamcinolone, confirming a continued preference for ranibizumab at higher-risk stages, though it is not a major difference.
So, at the end, we showed that both ranibizumab and triamcinolone are effective in treating diabetic retinopathy at various stages. This supports a preference for ranibizumab, as it seems in more advanced cases, due to its ability to prevent the growth of new blood vessels after laser treatment.
ROB assessment: The ROB assessment for the systematic review and meta-analysis on the use of the comparative ranibizumab with triamcinolone in the management of patients with DME includes evaluations of two studies.[1-5]
The assessment covers various categories of potential biases, including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. 6 studies exhibit a low ROB across most categories. Specifically, random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases were all rated as insignificant risk in both studies.
This consistent rating of minimal risk across these key domains suggests that the individual studies were conducted with robust methodologies, minimizing the potential for biased outcomes. In the overall assessment across the studies, many of the categories continue to reflect an insignificant ROB. Random sequence generation, allocation concealment, incomplete outcome data, selective reporting, and other biases were all reported with 100% insignificant risk. However, some concerns were noted in the categories of performance bias and detection bias, where approximately 16% of the assessments indicated an unclear ROB.
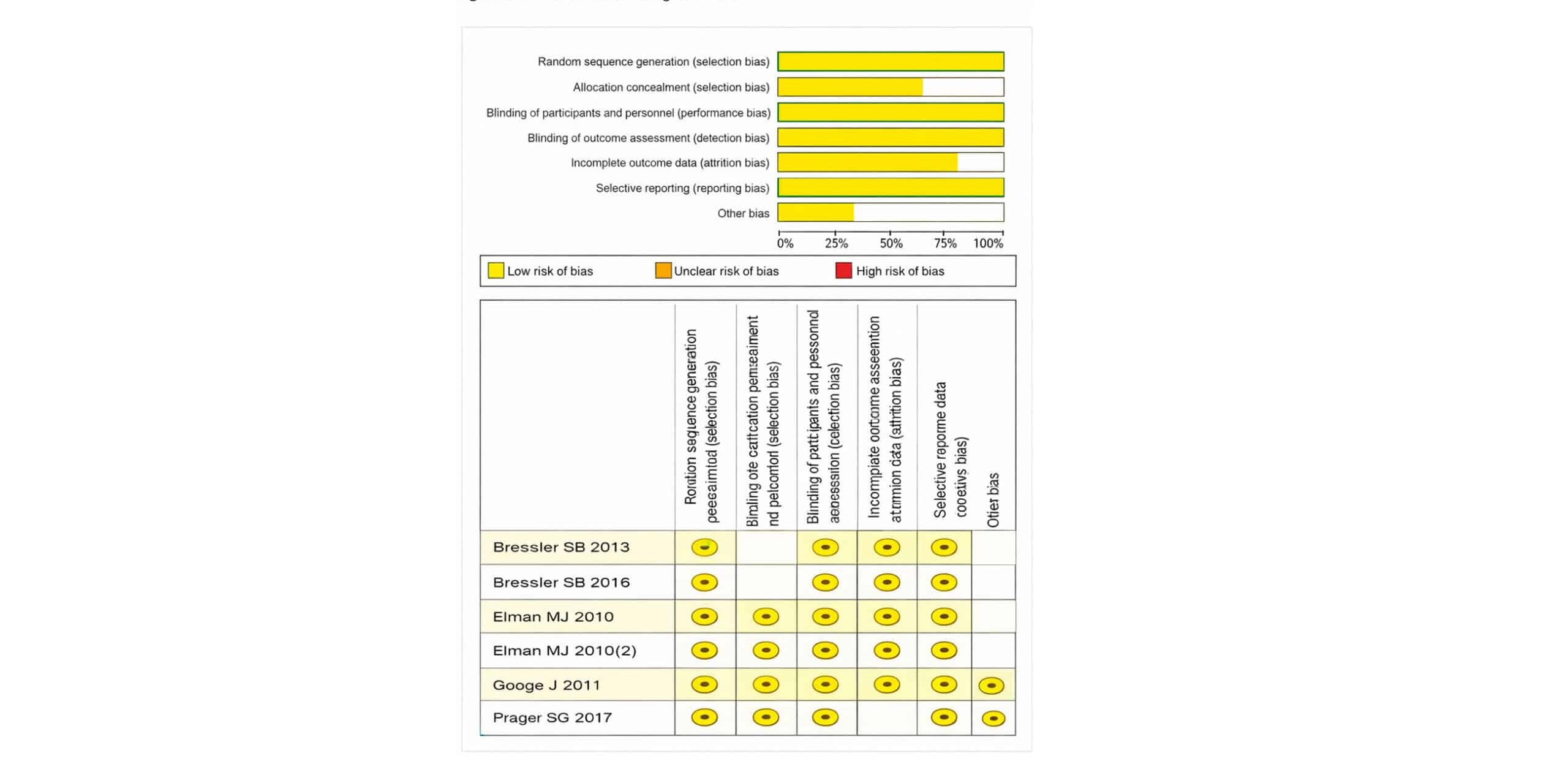
Figure 9: ROB assessment using RoB-2 tool
This ambiguity arises from the blinding of participants and personnel and the blinding of outcome assessment, which are crucial for preventing performance and detection biases. Overall, the studies demonstrate a strong methodological quality with minimal risk of bias, lending credibility to the findings of the systematic review and meta-analysis. Despite the minor concerns in performance and detection biases, the predominance of low-risk ratings supports the validity and reliability of the conclusions drawn regarding the effectiveness of ranibizumab and triamcinolone with prompt laser treatment in DME.
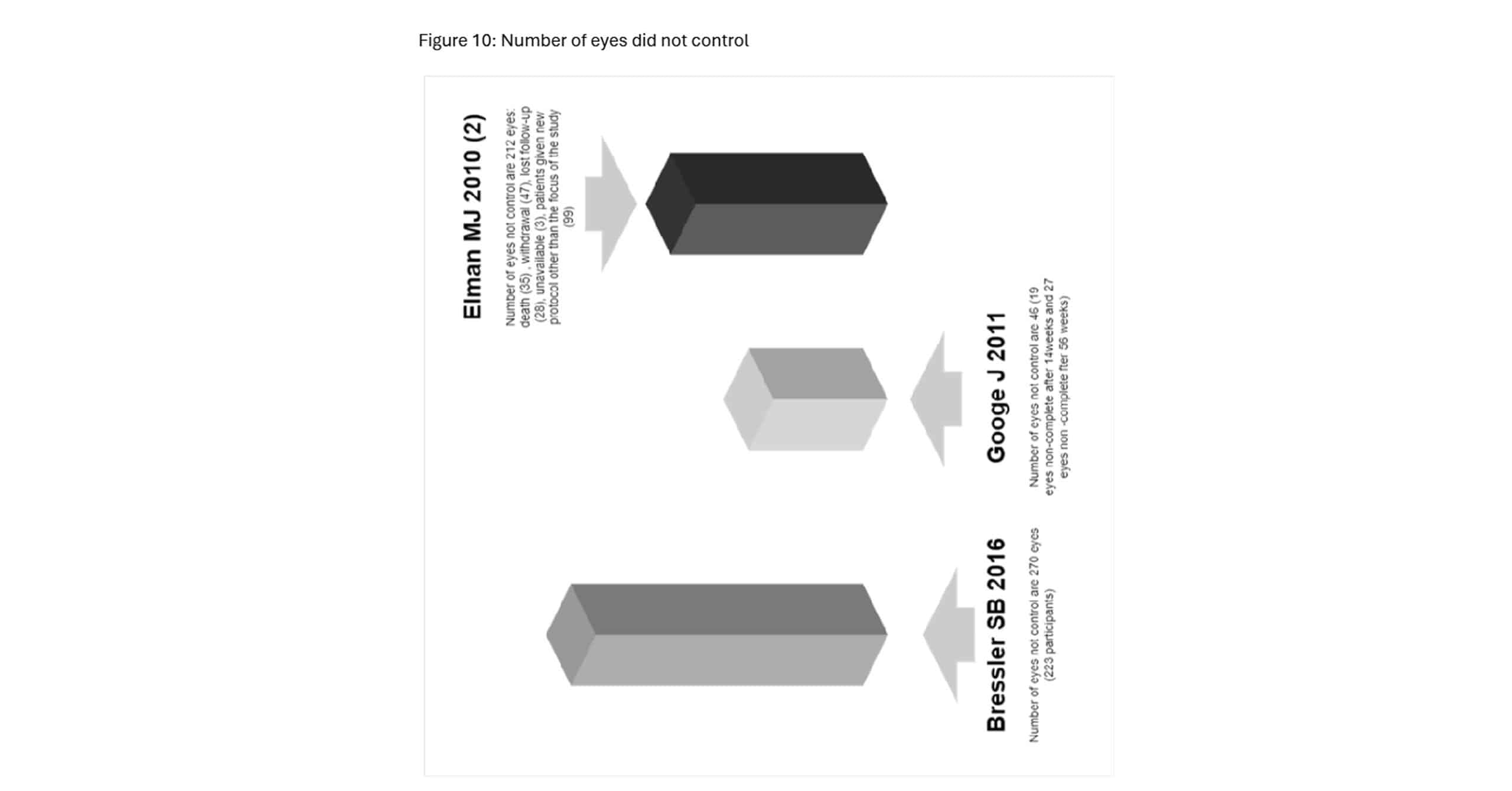
Figure 10: Number of eyes did not control
This image presents data from three studies that discuss the number of eyes that were not adequately controlled after treatment with ranibizumab and triamcinolone in patients with DME.
In the fourth study, a considerable number of patients (223) were not controlled, with a total of 270 eyes remaining uncontrolled after treatment. This study reported the highest number of uncontrolled cases compared to the other studies examined, indicating a larger proportion of patients whose DME management was insufficient despite the therapeutic interventions.[4] Another study reported a total of 46 eyes that were not controlled after ranibizumab or triamcinolone treatment. Specifically, 19 eyes were not controlled after 14 weeks of treatment, and an additional 27 eyes were not controlled after 56 weeks of follow-up. This study highlights the challenge of maintaining long-term control of DME in certain patients.[3] Similarly, one study found 212 eyes that were not adequately controlled by the end of the study. Of these, 35 patients died, 7 withdrew from the study, 28 were lost to follow-up, and 3 were unavailable for evaluation. Additionally, 99 eyes were placed on new treatment protocols, indicating that the original treatments were not effective in managing their DME.[4]
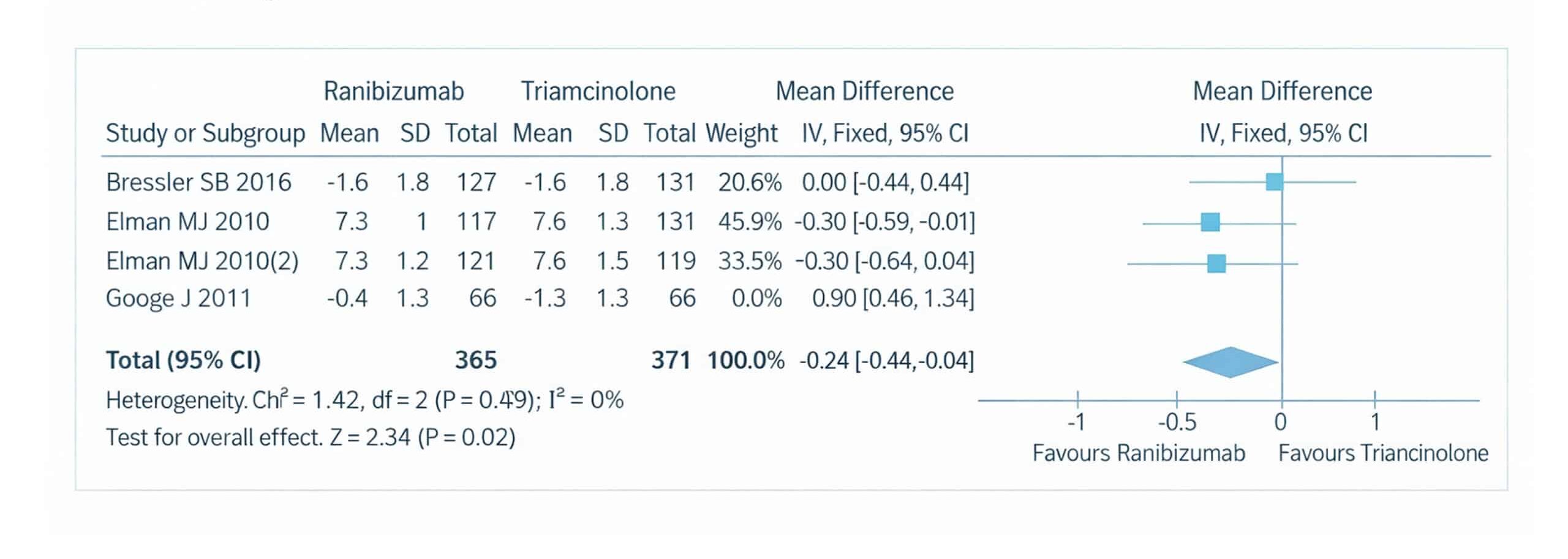
Figure 11: The forest plot of the OCT outcomes for patients with DME of the four included studies using a random effect model
The forest plot illustrates the results of a meta-analysis comparing the effectiveness of ranibizumab and triamcinolone in improving retinal volume OCT outcomes for patients with DME. This analysis includes data from four studies.[2-4] For each of these studies, the mean OCT measurements, standard deviations (SD), and total number of participants in both the ranibizumab and triamcinolone groups are provided. The analysis calculates the mean difference in OCT thickness between the two treatment groups, with 95% confidence intervals (CI) used to determine the precision of these estimates. The inverse variance method (IV) and a random-effects model were employed to account for potential variability between studies.
The weight assigned to each study in the meta-analysis is based on the sample size and variance, ensuring that studies with larger sample sizes or lower variance have a greater influence on the overall pooled estimate. To assess the consistency of the results across the included studies, the Chi-square (Chi²) test for heterogeneity was conducted, resulting in a p-value of 0.002 and an I² of 0%, indicating no significant heterogeneity. This suggests that the results across the studies were consistent and that any differences in OCT outcomes between ranibizumab and triamcinolone were not due to random variation between the studies. A sensitivity analysis was performed to further ensure that the overall effect size was duly influenced by any single study. This involved excluding one study at a time to verify the robustness of the pooled effect size. The overall mean difference between the ranibizumab and triamcinolone groups favoured ranibizumab, with a pooled effect size of 0.90 (95% confidence interval (CI): (0.46 to 1.34), p=0.02), although this result was not statistically significant. The homogeneity of the pooled studies was further confirmed by a Chi² value of 1.42, a p-value of 0.02, and an I² of 0%, indicating that the studies were similar in their findings, further reinforcing the consistency of the results.
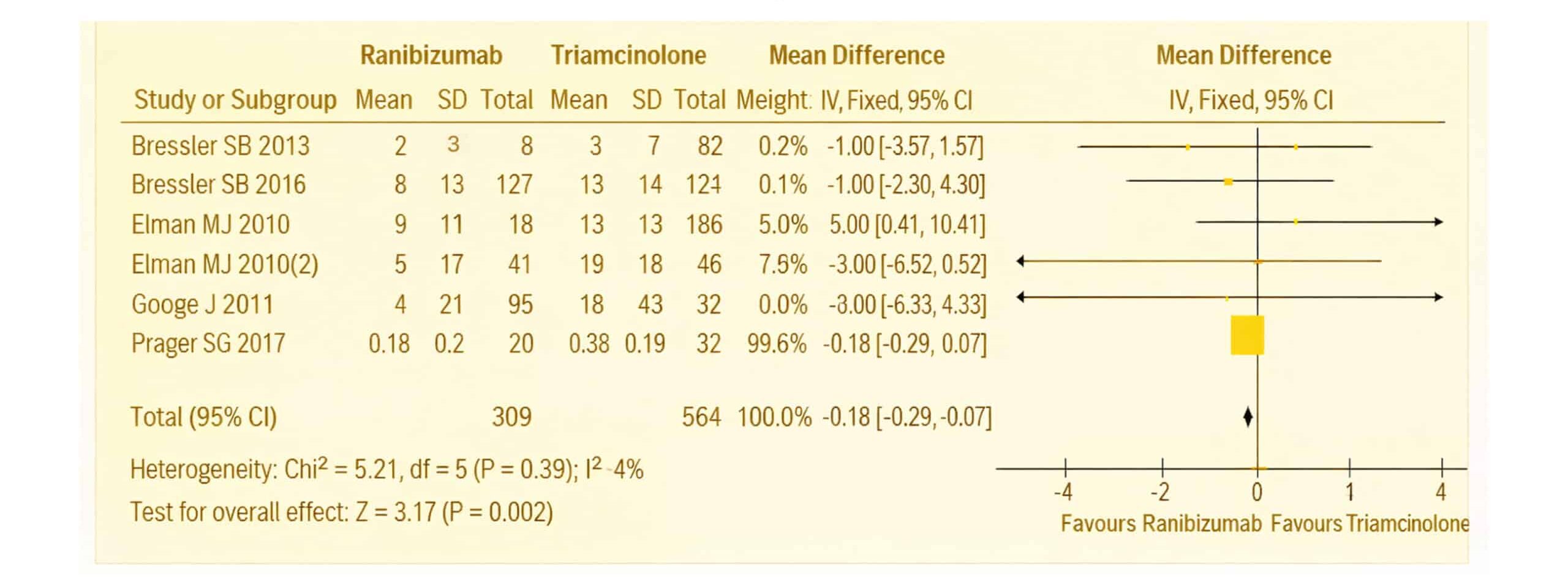
Figure 12: The forest plot of Visual acuity in patients with DME of the six included studies using a random effect model
The forest plot presents a meta-analysis comparing the effects of ranibizumab and triamcinolone on visual acuity in patients with DME. Data from six studies were analysed.[1-6] For each study, the mean, standard deviation (SD), and total number of participants in both the ranibizumab and triamcinolone groups are displayed. The analysis focused on the mean difference in visual acuity between these groups, with 95% CI provided to assess the precision of the results.
The IV method and a random-effects model were used to calculate the pooled mean differences, ensuring that the analysis accounts for any between-study variability. The weight assigned to each study in the meta-analysis was determined by its sample size and variance, ensuring that larger, more precise studies had a greater influence on the overall outcome. The heterogeneity of the studies was assessed using the Chi² test, which yielded a p-value of 0.002 and an I² of 4%, indicating no significant heterogeneity across the studies. This suggests the results were consistent and that the differences in visual acuity outcomes between ranibizumab and triamcinolone were not due to random variation.
To confirm the robustness of the findings, a sensitivity analysis was performed, excluding one study at a time to ensure that the overall effect size was not driven by any single study. The final pooled effect size was -0.18, with a 95% CI of (-0.29 to 0.07) and a p-value of 0.002. These results indicate that there was no statistically significant difference in visual acuity outcomes between ranibizumab and triamcinolone in patients with DME. Additionally, the homogeneity of the pooled studies was further confirmed by a Chi² value of 5.21, a p-value of 0.002, and an I² of 4%, reinforcing the consistency of the findings.
Symptoms for ranibizumab vs triamcinolone: DME develops when prolonged high blood sugar levels damage the blood vessels in the retina, the light-sensitive layer at the back of the eye, causing them to leak fluid. This fluid buildup in the macula, the main area of the retina that controls sharp vision, leads to swelling and results in vision impairment.
Treatment of DME: The treatment focuses on reducing retinal swelling, improving vision, and addressing the underlying cause of fluid leakage in the retina. The main treatment options include:
- Ranibizumab: Anti-VEGF drug that targets abnormal blood vessel growth and leakage. These drugs are injected directly into the eye and have been shown to improve vision and reduce macular edema.
- Triamcinolone acetonide: steroid drug that reduce inflammation and vascular permeability, which helps decrease fluid accumulation in the macula.
Ranibizumab, commonly used for the treatment of DME, has been associated with a range of systemic side effects, particularly affecting the cardiac, ophthalmic, and endocrine systems. Patients receiving ranibizumab have reported adverse effects such as hypertension (HTN) that developed due to congestive heart failure, where the heart is unable to pump blood efficiently, and peripheral edema, which involves swelling in the limbs due to fluid retention. Additionally, there have been instances of hypothyroidism, a condition where the thyroid gland fails to produce sufficient hormones, further complicating patient outcomes and impacts on the ophthalmic system by causing vitreous hemorrhage. The broad impact on critical organ systems highlights the importance of monitoring patients receiving ranibizumab for these potential complications.
In contrast, the side effects of triamcinolone, another treatment option for DME, appear to target different physiological systems, particularly hematological, cardiac, and ophthalmic health. Studies have documented significant increases in intraocular pressure (IOP), with some patients experiencing a rise of over 10 mmHg from baseline or IOP levels exceeding 30 mmHg. Additionally, patients undergoing treatment with triamcinolone are at an elevated risk of developing vitreous hemorrhage, but less than Ranibizumab, a condition where bleeding occurs into the eye’s vitreous humor, and anemia, a reduction in the number of red blood cells, which can lead to fatigue, pain in the extremities, and other symptoms. These findings emphasize the need for regular eye examinations and blood tests during triamcinolone therapy to mitigate these risks, patients receiving ranibizumab have reported adverse effects on the cardiac system, such as myocardial infarction.
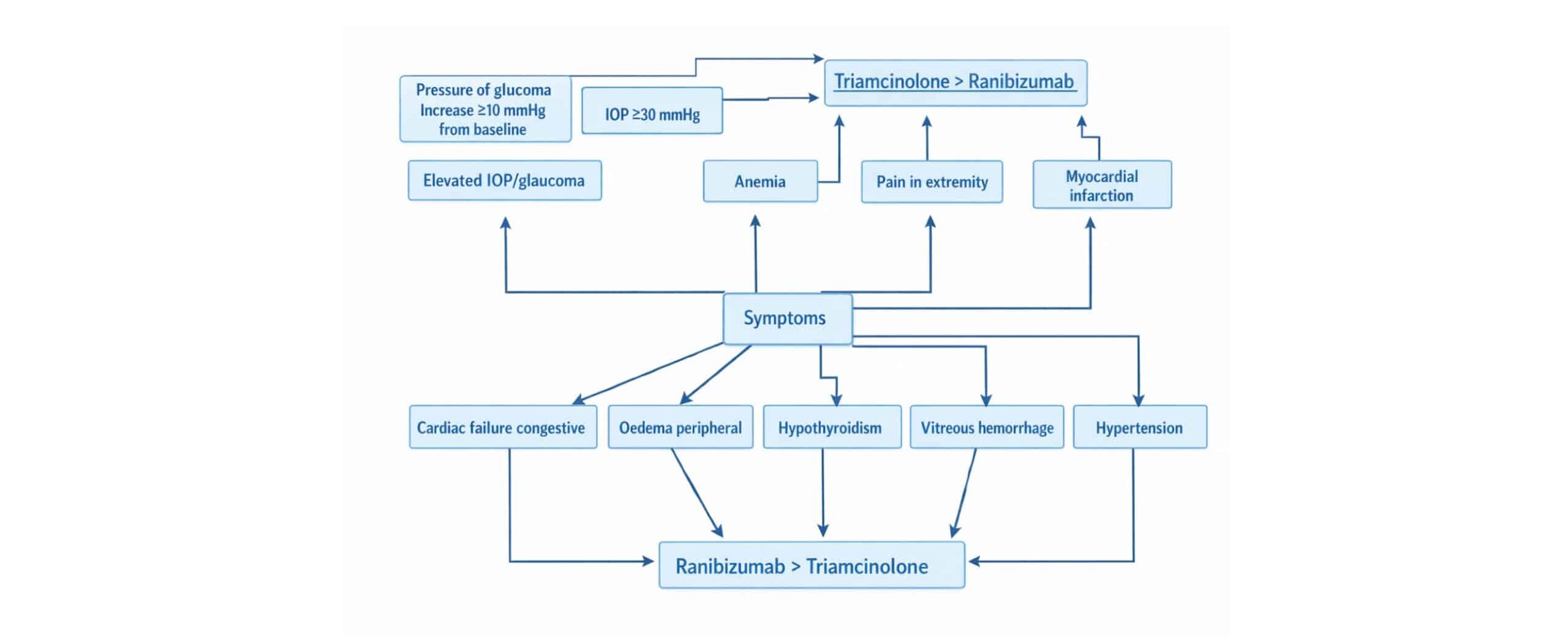
Figure 13: Symptoms for patients taken ranibizumab vs triamcinolone
In our study, we observed a distinct difference in the adverse effects experienced by patients receiving ranibizumab compared to those receiving triamcinolone. Elevated IOP > 30 mmHg was significantly more common among patients treated with triamcinolone (25%-27%) than in those treated with ranibizumab (1%-2%). Similarly, the incidence of pressure increases greater than 10 mmHg, which is associated with the risk of glaucoma, was notably higher in the triamcinolone group (22%- 42%) compared to the ranibizumab group (3%-9%).
On the other hand, vitreous hemorrhage was more frequently observed in patients treated with ranibizumab (6%-23%) than in those treated with triamcinolone (4%-18%). The occurrence of cataracts was also more prevalent in the triamcinolone group, with a rate of 6%-15%, compared to the ranibizumab group, where cataracts were observed in 3%-5% of patients. Furthermore, HTN was a common adverse effect in both treatment groups. However, it was more pronounced in the ranibizumab group, with 82% of patients affected, while 73% of the triamcinolone group experienced this side effect.
This comparison highlights the differing safety profiles of ranibizumab and triamcinolone, providing important considerations for clinicians when choosing treatment for DME based on patient-specific risk factors.
Discussion
Our meta-analysis aimed to evaluate the effectiveness and safety of ranibizumab, an anti-VEGF agent, and triamcinolone, a corticosteroid, in the management of DME with prompt laser treatment administered to both groups. The analysis included patients at various stages of diabetes and documented outcomes such as the number of eyes that could not be controlled by treatment and patient mortality rates. Importantly, OCT findings favoured ranibizumab over triamcinolone, suggesting that ranibizumab may provide better structural outcomes for patients in terms of retinal thickness. However, visual acuity outcomes, particularly related to retinal volume, did not show a significant advantage for either drug. Visual acuity was compared using ETDRS scores, with patients categorized based on their stage of diabetic retinopathy, with NPDR and PDR.
One of the notable aspects of our study was the comparison of systemic and ocular side effects between the two drugs. Patients who received ranibizumab experienced a higher incidence of systemic side effects, including HTN, hypothyroidism, vitreous hemorrhage, and congestive heart failure. These findings are particularly important given the systemic nature of DME and the complex comorbidities often present in diabetic patients. On the other hand, triamcinolone-treated patients exhibited a higher risk of ocular complications such as elevated IOP, glaucoma, cataracts, anemia, and myocardial infarction (MI). These findings highlight the need for a tailored approach when selecting treatments based on individual patient profiles and preexisting conditions.
| Side effects | Percents of patients taken ranibizumab and shown side effect |
Percents of patients taken triamcinolone and shown this side effect |
| IOP ≥30 mmHg | 1%-2% | 25%-27% |
| Pressure Increase
≥10 mmHg (glaucoma) |
3%-9% | 22%-42% |
| Vitreous
hemorrhage |
6%-23% | 4%-18% |
| HTN | 82% | 73% |
| cataract | 3%-5% | 6%-15% |
Table 3: Percentage of patients shown adverse effect after taken ranibizumab and triamcinolone
The analysis of disease duration also played a critical role in our study. We included data on how the duration of diabetes influenced treatment outcomes, and this was further divided into distinct stages of the disease. This allowed us to better understand how the chronicity of diabetes affected the response to treatment in patients with DME. Additionally, we evaluated the BCVA after 12 months of treatment, offering insights into the long-term efficacy of both ranibizumab and triamcinolone in preserving or improving vision in patients with DME. This data is crucial for clinicians aiming to predict patient outcomes over an extended period and to make informed decisions about ongoing treatment strategies.
Despite these comprehensive findings, our study faced some limitations. We were unable to include a comparison of changes in visual acuity in eyes that were pseudophakic at baseline. Since the presence of a pseudophakic lens can significantly influence visual outcomes, particularly in older diabetic patients, this omission limits the generalizability of our results to this subset of patients. Additionally, our study lacked data on OCT central subfield thickness, which could have provided more precise measurements of macular edema and further clarified the differences between ranibizumab and triamcinolone in terms of anatomical outcomes.
These limitations highlight the need for future research to focus on specific subgroups of patients, such as those with pseudophakic eyes, and to include more detailed OCT assessments to better understand the structural changes associated with both treatments. More robust data on these areas will help confirm or refine our findings and offer clearer guidance on the most effective treatment strategies for DME. Overall, our meta-analysis contributes valuable insights into the management of DME, particularly in terms of safety profiles and disease outcomes, and underscores the importance of individualized treatment approaches based on patient characteristics and comorbidities.
This meta-analysis faced several limitations that impact the strength and generalizability of its findings. One major challenge is the heterogeneity of treatment protocols across the included studies, particularly in patients with DME. The variability in patient characteristics, stages of diabetes, and treatment approaches made it difficult to draw definitive conclusions about the comparative efficacy of ranibizumab and triamcinolone. This inconsistency underscores the need for more standardized study designs to better evaluate these drugs across the spectrum of complex DME cases.
Furthermore, the analysis was constrained by the limited number of studies included, specifically only six that provided data on visual acuity using the ETDRS scoring system and OCT measurements of retinal volume. This small sample size restricts the statistical power of the findings, limiting the ability to detect more nuanced differences between the two treatments. Additionally, the absence of sufficient data comparing changes in visual acuity in pseudophakic eyes at baseline and OCT central subfield thickness further reduces the comprehensiveness of the analysis.
These limitations highlight the urgent need for further research. Future studies should aim to provide more detailed comparisons of these drugs, particularly in specific subgroups such as pseudophakic patients, and include more robust OCT-based assessments. Expanding the scope and quality of available data will help confirm these preliminary findings and allow for more definitive. Conclusions regarding the effectiveness and safety profiles of ranibizumab and triamcinolone in the treatment of DME.
Conclusion
In conclusion, this meta-analysis compared the efficacy and safety of ranibizumab and triamcinolone in the management of DME. Ranibizumab demonstrated more favourable outcomes in terms of OCT measurements, indicating better structural improvements in the retina compared to triamcinolone. However, patients in the ranibizumab group experienced a higher incidence of systemic side effects, including HTN, vitreous hemorrhage, and congestive heart failure, whereas triamcinolone was associated with ocular complications such as elevated IOP, glaucoma, cataracts, and myocardial infarction. Additionally, disease control was less effective in the ranibizumab group, as a higher number of eyes were unresponsive to treatment compared to the triamcinolone group. These findings highlight the need for individualized treatment strategies that consider both the efficacy and safety profiles of each drug, considering patient-specific risks and conditions.
References
- Prager SG, Lammer J, Mitsch C, et al. Analysis of retinal layer thickness in diabetic macular oedema treated with ranibizumab or triamcinolone. Acta Ophthalmol. 2018;96(2):e202-208. doi:10.1111/aos.13520
PubMed | Crossref | Google Scholar - Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. doi:10.1016/j.ophtha.2010.02.031 PubMed | Crossref | Google Scholar
- Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609-614. doi:10.1016/j.ophtha.2010.12.033 PubMed | Crossref | Google Scholar
- Googe J, Brucker AJ, Bressler NM, et al. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina. 2011;31(6):1009-1027. doi:10.1097/IAE.0b013e318217d739
PubMed | Crossref | Google Scholar - Bressler SB. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130(9):1153-1161. doi:10.1001/archophthalmol.2012.1107 PubMed | Crossref | Google Scholar
- Bressler SB, Qin H, Melia M, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131(8):1033-1040. doi:10.1001/jamaophthalmol.2013.4154 PubMed | Crossref | Google Scholar
Acknowledgments
Not reported
Funding
Not reported
Author Information
Corresponding Author:
Mo’men Shabib
Department of Medicine
Yarmouk university, Jordan
Email: moumenaldabobe@yahoo.com
Co-Authors:
Ameen Mahmoud, Mohammad Al-Sharab, Ahmed Al-Karasneh, Osama Al-Ramahi
Department of Medicine
Yarmouk university, Jordan
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
This meta-analysis was conducted using data from previously published studies, all of which were ethically approved by their respective institutional review boards. As the analysis involved secondary use of aggregated and anonymized data from these studies, no new patient recruitment or data collection was required. Therefore, obtaining additional patient consent was not necessary for this research. All original studies included in this analysis followed ethical guidelines and standards for the protection of human subjects.
Conflict of Interest Statement
Not reported
Guarantor
None
DOI
Cite this Article
Momen S, Ameen M, Mohammad AS, Ahmed AK, Osama AR. Comparative Safety and Effectiveness of Ranibizumab and Triamcinolone with Prompt Laser Treatment in Diabetic Macular Edema: A Systematic Literature Review and Meta-Analysis. medtigo J Pharmacol. 2024;1(1):e3061124. doi:10.63096/medtigo3061124 Crossref









