Author Affiliations
Abstract
Metabolic Syndrome (MS) is a metabolic disorder often presented as a cardiovascular risk factor, with central fat accumulation resulting in insulin resistance (IR). MS represents a cluster of metabolically related symptoms such as obesity, hypertension, dyslipidemia, and carbohydrate intolerance, significantly increasing the risk of Type II diabetes mellitus (T2DM). Insulin resistance and hyperinsulinemia are consistent characteristics of MS, but which of these features constitutes the initiating insult is still widely debated. This study aims to understand the biochemical changes in patients with metabolic syndrome. After analyzing data from different studies, it is concluded that the biochemical changes in metabolic syndrome are complex, multi-system changes which include IR, dyslipidemia, inflammation, hypercoagulable state or thrombosis, oxidative damage, adipokine dysregulation, and hormonal impairment. This syndrome can be managed through behavioral therapy (diet & exercise, lifestyle modification, and proper training), medical therapy (pharmacotherapy), and surgical procedures (metabolic surgery/bariatric surgery (BS)).
Keywords
Type-II diabetes mellitus, Metabolic syndrome, Dyslipidemia, Hyperinsulinemia, Insulin resistance, Obesity.
Introduction
MS is a complex metabolic disorder characterized by insulin resistance, abdominal obesity, hypertension, and hyperlipidemia. MS mainly causes three disturbances: lipid metabolism, carbohydrate metabolism, and hemodynamic disturbance, resulting in obesity & dyslipidemia, glucose intolerance & diabetes, and arterial hypertension, respectively.[1-8] There are different standard definitions of metabolic syndrome according to various expert groups such as World Health Organization (WHO), National cholesterol education program adult treatment panel III (NCEP ATP III), European group for the study of insulin resistance (EGIR), American association of clinical endocrinologists (AACE), and the International diabetes federation (IDF). For instance, central obesity is necessary for the IDF criteria but not for the EGIR, NCEP ATP III, and WHO criteria.[1,3,5] It is estimated that 16% of people worldwide suffer from MS who are over 20 years old.[2] The prevalence of metabolic syndrome has increased in developing Western and Asian countries.[1,3] The prevalence of metabolic syndrome in China rose from 24.2% in 2015 to 31.1% in 2017.[4,7] It is found that MS patients have mortality rates twice as high, suffer from heart attacks/strokes three times more, and suffer from T2DM five times more compared to the normal population.[6,8,10] MS increases the risk of developing T2DM and cardiovascular diseases; it is a strong predictor for the development of cardiovascular diseases and T2DM.[1,3,8,9] Both T2DM and MS are metabolic disorders due to IR.[1,11,12]. The degree of β-cell dysfunction is associated with the severity of MS.[13] The pathogenesis of MS includes both genetic and acquired factors, which ultimately result in an inflammatory process.[14]
Disease
MS is a metabolic disorder often presented as a cardiovascular risk factor, with central accumulation of fat resulting in IR.[15] It is a multifactorial disease defined as a cluster of mainly these four conditions: obesity (which may be central or visceral), having IR with hyperinsulinemia, hyperamylasemia, and hyperleptinemia due to leptin resistance; hyperlipidemia with atherogenic dyslipidemia, which is characterized by a high level of very low-density lipoproteins (VLDL) or triglycerides (TGs), a high level of low-density lipoprotein (LDL) cholesterol (LDL-C), and a low level of high-density lipoprotein (HDL) cholesterol (HDL-C); essential hypertension (HTN); and glucose intolerance with or without T2DM. That is, MS is a collection of unhealthy body parameters with laboratory abnormalities such as atherogenic dyslipidemia, hypertension, glucose intolerance, a pro-inflammatory condition, and a pro-thrombotic condition.[16-21]. Clinical picture and biochemical changes with complications are explained in Figure 1.
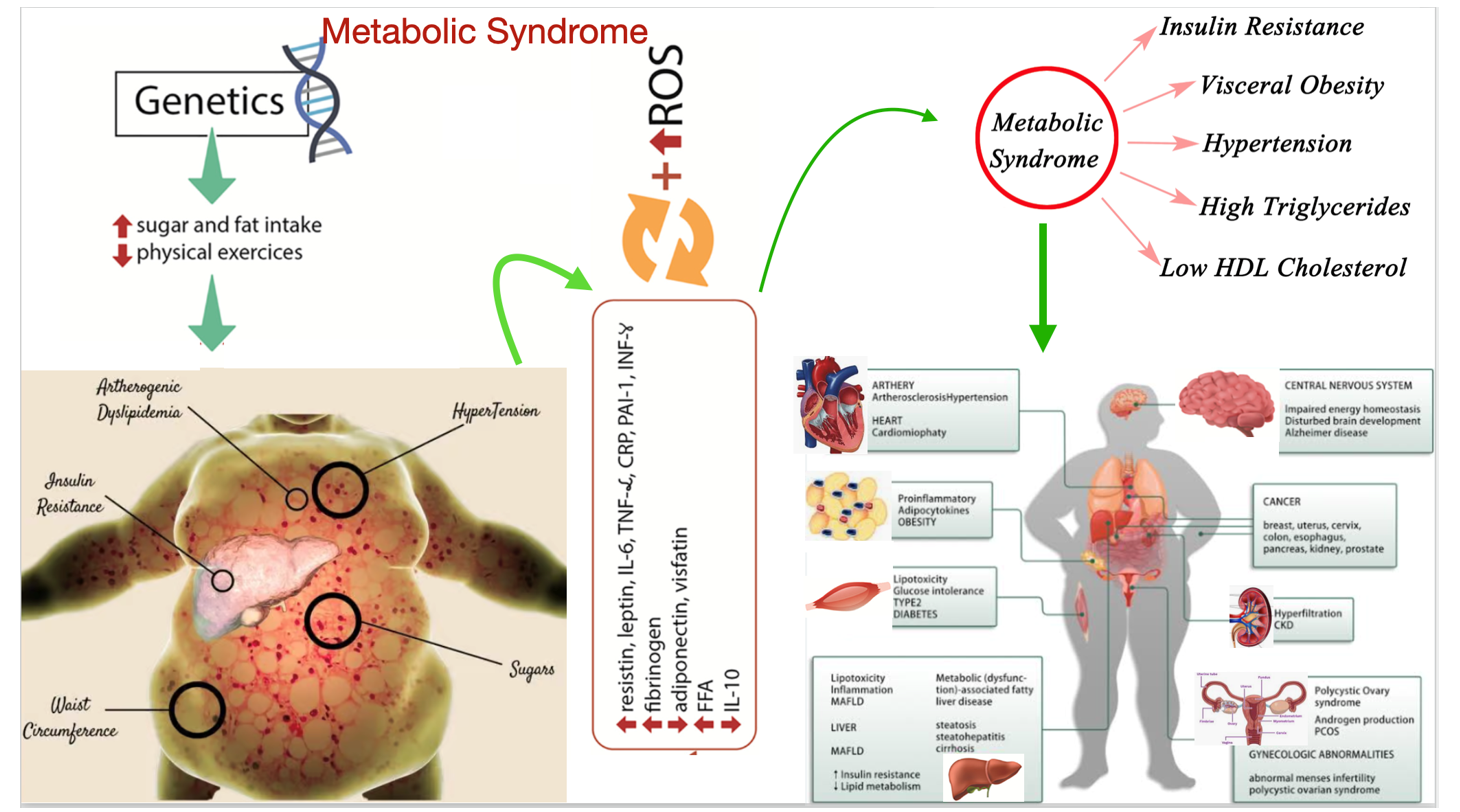
Figure 1: Photogenic diagram shows the clinical presentation and biochemical changes with its complications
Abbreviations: Interleukine-6(IL-6), tumor necrotic factor-𝛼 (TNF-𝛼), C-reactive proteins (CRP), free fatty acid (FFA), plasminogen activator inhibitor-1(PAI-1), interferon-Y (IFN-Y), metabolic dysfunction -assocaited fatty liver disease (MAFLD), chronic kidney disease (CKD), polycystic ovarian syndrome (PCOS)
Pathophysiology and mechanism
The pathophysiological mechanism of MS illustrates a metabolic disorder resulting from obesity with IR secondary to a chronic inflammatory condition.[22,23] Changes in visceral vascular and lymphatic secondary to high visceral adipose tissue caused hypoxia and lipotoxicity of adipocytes, which are accompanied by the release of fatty acids and other substances, which activate the pro-inflammatory pathways of the parenchymal cells of the tissues.[24] This inflammatory process occurred at multiple sites of adipose tissue, causing the systemic spread of inflammatory cytokines to visceral organs, especially which is sensitive to insulin, resulting in the accumulation of lipids in the muscles and liver that predispose to IR and play an important role in the progression and transition of MS to cardiovascular diseases (CVD) and T2DM.[25,26] It was found that high levels of glucose due to IR caused high insulin levels (Hyperinsulinemia) by stimulating pancreatic β cells, and this compensatory effect in multifactorial and initial to maintained euglycemic state, but if continuous hyperglycemic and hyperlipidemic condition persist then leads to β cells dysfunction and/or death which leads to diabetes.[13,19,27] IR due to abnormal adipocyte cells causes inhibition of insulin-mediated lipolysis and leads to increases in FFA, which further inhibits the anti-lipolytic effect of insulin and can inhibit the insulin-dependent glucose uptake.[19,28] Visual of the pathophysiological mechanism as shown in Figure 2.
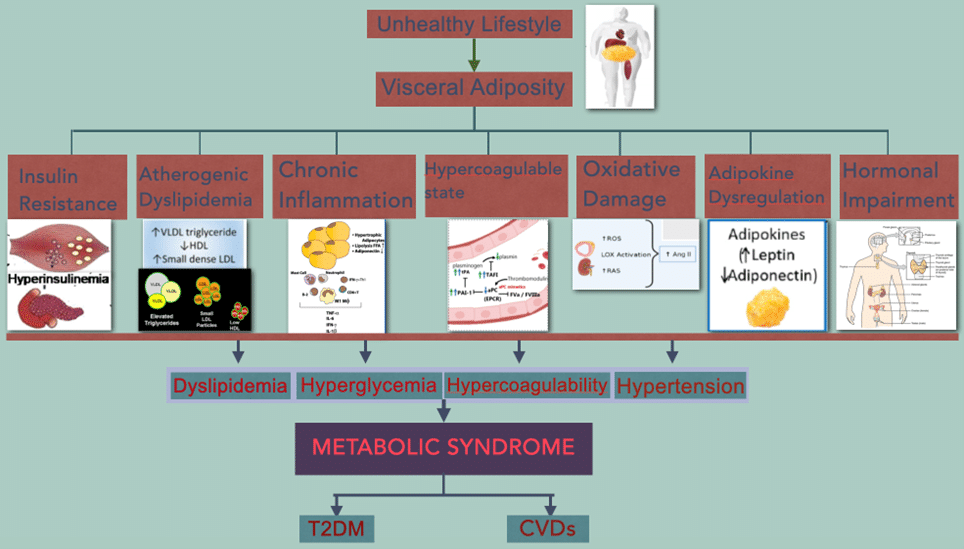
Figure 2: Visual diagram of the pathophysiological mechanism of metabolic syndrome with common complications
Abbreviations: Plasminogen activator inhibitor-1 (PAI-1), tissue plasminogen Activator (tPA), reactive oxygen species (ROS), renin angiotensin system (RAS), angiotensinogen-II (Ang-II)
Diagnostic criteria
MS has been defined a bit differently by different expert groups like WHO, IDF, EGIR & NCEP ATP, and hence there was a difficulty in diagnosing. Then, the joint statement approved a consensus definition to help diagnose if three of the following criteria are present.[5,19]
- High waist circumference (WC): Definition depends upon country& population ( ≥ 102cm and ≥88cm in European male and female respectively)
- Blood triglyceride (TG): ≥ 150mg/dL
- Blood HDL-cholesterol: < 40mg/dL in male & < 50mg/dL in female
- High blood pressure (BP): ≥ 130/85mmHg
- Fasting blood glucose (FBG): ≥ 100mg/dL
Biochemical changes
Biochemical changes that occur in MS are complex, and a few important ones are explained below.
IR: During pathophysiological conditions, normal insulin levels don’t adequately respond to peripheral target tissues such as adipose tissues, muscle, and liver, where pancreatic β cells produce more insulin to overcome hyperglycemia.[22] Compensatory hyperinsulinemia due to IR results from multiple impairement occurs in MS, which may occur due to impaired insulin signaling of the phosphoinositide 3-kinase/ Protein kinase B (PI3kinase/AKT) pathways which is associated with serine hyperphosphorylation of the insulin receptor substrate 2 (IRS2) signaling pathway where low insulin level stimulated no production with decreased vasodilation and pro constrictive, prothrombotic along with pro-atherosclerotic effect on vessels resulting in atherosclerosis.[9,29,30] Hormone leptin and Adipocyte-derived peptide adipokine play an important role in adiposity. IR and leptin also play an important role in insulin-mediated glucose uptake.[9,31,32] Leptin plays a role in volume and BP regulation under normal physiological conditions, while leptin resistance (LR) and hyperleptinemia occur in obese patients with MS, which, along with adipokines, play an important role in the development of HTN and cerebro-cardiovascular disease (CCVD).[33] High compensatory insulin and amylin may increase the risk of islet amyloid deposition secondary to amyloidogenic hyperamylasemia and hyperinsulinemia, where these two conditions are common to IR with MS. The study showed that obese mice developed IR in fat and muscle due to blockage of NF-kB and Jun N-terminal kinase (JNK) pathway, which play an important role in the suppression of inflammatory signals.[34]
Dyslipidemia: Dyslipidemia is defined as quantitative lipid abnormalities that cause structure, metabolism, and biological activities of both atherogenic lipoproteins and antiatherogenic HDL-C, which includes high levels of lipoproteins containing apolipoprotein B (ApoB), high levels of TGs, high levels of small particles of LDL, and Low levels of HDL-C.[22] Oxidative LDL (Ox-LDL) plays an important role in the atherosclerosis process by expressing adhesive molecules, cytokines, and growth factors along with changes in the function of vasoactive molecules such as NO, AngII, or endothelin 1 (ET1).[34,35] High cholesterol level promotes oxidative stress in endothelial cells.[35,36] In the mice model, it was found that FFA can activate the RAS in mice adipocytes (3T3L1) by TLR4/NF-kB pathway.[36,37] Insulin is the regulator of lipoprotein lipase, which is the mediator of VLDL clearance; therefore, there is hypertriglyceridemia in IR conditions due to high VLDL production and low clearance.[22] TGs of VLDL transform into HDL in the presence of cholesterol ester transport protein (CETP) in exchange for cholesteryl esters, which finally transform into TG-enriched HDL and cholesteryl ester-enriched VLDL.[22]
Inflammation: The immune system and metabolic system play an important role in metabolic homeostasis, while an imbalance between these two systems causes mild chronic systemic inflammation, which leads to MS.[38,39] Chronic inflammation is characterized by the abnormal presence of abnormal cytokines, which causes an increase in the acute reactant and another medium which is closely associated with various chronic diseases such as obesity, T2DM, atherosclerosis, non-alcoholic fatty liver disease, gout, and so on.[38-40] Several scholars suggested that inflammatory response plays a role in IR and glucose intolerance, which may induce β cell dysfunction and promote tissue remodeling.[40,41] T-cells play an important role in inflammation and are directly involved in the development of metabolic diseases such as chronic inflammation due to the balance of T17/Treg is closely related to metabolic disease, T-cell phenotype stratifies the obese and /or T2DM, which exhibits positive therapeutic and prognostic implications and also T cell senescence plays a role in hepatic glucose homeostasis.[38,42-44] TNF-𝛼 induces adipocyte apoptosis and promotes IR by inhibiting insulin receptor substrate, one signaling pathway whose value increases with an increase in body weight, WC, and TGs while value decreases with increased HDL-C level.[45] High CRP is associated with increased WC, IR, high body mass index (BMI), hyperglycemia, and increased metabolic components where, whereas CRP is also an independent predictor of the occurrence of CVD, regardless of MS.[46] Both Adipose tissue and skeletal muscle in human are responsible for the release of IL-6, which has both inflammatory and anti-inflammatory actions.[22] IL-6 receptors are also present in different régions of brain, such as in the hypothalamus, where it controls an appetite and energy intake.[22] IL-6 is a systemic adipokine that impairs insulin sensitivity and is a determinant of the production of CRP by hepatic tissue, where its value increases with an increase in BMI, high fasting insulin, and T2DM while a decrease in an increased level of HDL-C level.[22]
Hypercoagulable state or thrombosis: A prothrombotic state is characterized by impairment of pro-coagulation factors such as an increase in fibrinogen, factor VII, and factor VIII, as well as the PAI-1, platelet abrasions, and endothelial dysfunctions.[22] A Grundy study found that fibrinogen and an acute-phase reactant have a positive association with a high-cytokine state.[47] PAI-1 is a serine protease inhibitor that is secreted from intra-abdominal adipocytes, platelets, and the vascular endothelium, which exerts an effect on inhibition of tPA and is hence considered a marker of dysfunctional fibrinolysis and atherothrombosis, where it depends on abdominal obesity and inflammatory conditions, and hence predicts the risk of thrombosis and adverse cardiovascular outcomes.[22,48] Adiponectin plays a role in multifactorial anti-atherogenic action that includes inhibition of endothelial activation, reduction in conversion of microphage to foam cells, and inhibition of smooth cell proliferation and arterial remodeling, which implies the development of mature atherosclerotic plaque.[22] Adiponectin is inversely related to CVD risk factors such as blood pressure, LDL-C, and TGs.[49]
Oxidative damage: Oxidative stress (OxS) is the cause and consequence of MS, which triggers the signaling pathways dysregulation associated with metabolism and epigenetics, including micro ribonucleic acid (microRNA), which are biomarkers of metabolic disorders.[35] OxS is a prolonged condition of impairment between the oxidative and anti-oxidative systems of cells caused by the overproduction of free radicals and ROS, resulting in cellular and tissue damage.[35,50] The peptide exerts its pathogenic effect by activating nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase, which increases the production of ROS.[51] Mitochondria are a great source of ROS generation from the electron transport chain (ETC) due to the leak of a few high-energy electrons and their direct reaction with oxygen.[52] Mitochondria play an important role in effective antioxidant mechanisms, which activate superoxide dismutase and other enzymes to kill ROS produced either locally or with the help of other organelles, such as peroxisomes.[53] The abnormal mitochondrial function may lead to the overproduction of ROS, causing cell injury, i.e., Oxidative stress, which may contribute to many metabolic disorders, including MS.[54] Mitochondrial function declined due to Insulin IR may exhibit susceptibility to oxidative stress and reduced oxidative phosphorylation and energy production (EP).[55] ROS has several pleiotropic effects, including endothelial injury, NF-kB expression, aggregation of platelets, LDL oxidation, and expression of lipoprotein receptor-1(LOX-1) on vascular smooth muscle cells (VSMCs) and endothelium. RAS, LOX-1, and ROS together give a positive feedback loop that induces a variety of cycles of endothelial dysfunction, inflammation, and fibroblast proliferation, resulting in the progression of dyslipidemia, T2DM, hypertension, vasculopathy, and CVDs.[51]
Adipokine dysregulation: White adipose tissue (WAT) is composed of Adipose-derived stem cells (ASCs), preadipocytes, adipocytes, and immune cells that are responsible for fat storage and deposit an excess of energy in mobilized FA or TGs according to metabolic needs.[35] WAT works as an immunological organ that releases peptides (Adipo-/cytokines) metabolites capable of producing systemic actions, including body weight /energy balance, appetite regulation, glucose homeostasis, insulin signaling, and blood pressure control. Hypoxia has an inciting etiology of necrosis and macrophage infiltration into adipose tissue, which leads to an overproduction of biologically active metabolites called adipocytokines that include glycerol, FFA, pro-inflammatory mediators (TNF-𝛼, IL-6, PAI-1, CRP).[56-58] Leptin is the first known adipocyte hormone whose genetic absence causes massive obesity and suppresses appetites, while other hormones like adiponectin have negative effects [59,60]. Adipocytokines transmit endocrine, autocrine, and paracrine signals to proceed with multiple processes, including insulin sensitivity, oxidant stress, energy metabolism, blood coagulation, and inflammatory responses that are thought to accelerate cardiovascular disorders such as atherosclerosis, plaque rupture, and atherothrombosis.[22]
Hormonal impairment: Chronic hypersecretion of stress hormones like cortisol when exposed to a permissive environment in individuals with genetic predisposition, which may lead to visceral fat accumulation due to chronic hypercortisolism, low growth hormone secretion, and hypogonadism.[61] Cortisol increases the activities of fatty acid synthesis and promotes the secretion of lipoproteins, induces the hepatic gluconeogenesis pathway, promotes the differentiation of pre-adipocytes to adipocytes that increase body fat mass, inhibits insulin-stimulated amino acid uptake by adipocytes, and causes high lipolysis or oxidation of lipid, which causes peripheral IR. Cortisol secretion and clearance have a positive relation with fasting glucose, blood pressure, and insulin.[62] These hormonal abnormalities may lead to hypersecretion of reactive insulin, visceral obesity increases, and sarcopenia, which leads to dyslipidemia, hypertension, and T2DM.[22,63]
Comorbidities and complications
MS increases the risk of CVDs due to the presence of pro-inflammatory conditions, which may result from high plasma glucose levels.[64] Diabetes is a serious health threat and the leading cause of CVDs, end-stage renal disease (ESRD), and blindness, which often arises in the background of MS.[65] Pre-diabetic has clinically asymptomatic conditions but increase the risk of T2DM and CVDs, which can be limited by lifestyle modification and correction of other risks associated with cardio-metabolic disease.[66]. Obesity is one of the risk factors for MS, including HTN, T2DM, dyslipidemia, and non-alcoholic fatty liver disease (NAFLD) that may lead to many complications such as obstructive sleep apnea (OSA), which contributes to low-quality of life and high cardiovascular disorders and also associated with osteoarthritis, increased risk of cancer and cancer-related mortality that may attributable to chronic hyperinsulinemia.[67-70]. Intra-vascular and extra-vascular fatty deposition increases the risk of CVDs and other morbid conditions, while subcutaneous fat deposition in the lower body is seen to be metabolically healthy and less prone to developing metabolic comorbidities.[71,72]. HTN is a critical risk factor for CVDs, which is one of the factors of MS that occurs five times more commonly with visceral obesity than with normal body weight, and HTN represents the leading cause of worldwide premature death.[73,74]. Dyslipidemia is one of the factors of MS that is responsible for significant morbidity and mortality, and that can be limited by lowering LDL-C with therapy.[75,76]. MS may also be complicated as NAFLD, major depressive disorder (MDD), male infertility may be due to oxidative stress on sperm quality, polycystic ovarian syndrome (PCOS), cognitive impairment (Alzheimer’s disease and vascular dementia).[77,78]
Treatment
MS can be treated by behavioral therapy, pharmacotherapy, and surgical therapy, which are as follows:
Behavioral therapy: Behavior therapy includes a set of principles and techniques that modify eating and physical activity.[79] Lifestyle modification, low-calorie with low-fat diet (Mediterranean diet), and increased physical activity play important roles in the prevention and limiting the progression of the disease, with risk reduction.[22,51]
Pharmacotherapy: The national institutes of health guidelines for the management of obesity and comorbidities associated with it, which include appetite suppression and nutrition absorption inhibitors.[80] Statins therapy for the management of dyslipidemia.[51] Anti-hypertensive therapy like beta-blockers (β-blockers), calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), anti-hyperglycemic agents like metformin, dipeptidyl peptidase-4 (DPP-4), Glucagon-like peptide-1 GLP-1), and anti-platelet therapy to reduce comorbidities associated with hypercoagulable state.[22,51]
Surgical therapy: BS is a metabolic surgery that is indicated to reduce comorbidities. It is indicated in patients with extreme obesity (high BMI >40 kg/m2) or if they have a BMI >35 to 40 kg/m2 with one or more comorbidities.[80] BS can be divided into three types according to their mechanism of action. They are restrictive types (adjustable gastric banding, vertical banded gastroplasty, and sleeve gastrectomy), malabsorptive types (biliopancreatic diversion and biliopancreatic diversion with duodenal switch), and a combination of both (Roux-en-Y gastric bypass).[81]
Conclusion
MS is a complex metabolic disorder that can be characterized by insulin resistance, abdominal obesity, hypertension, and hyperlipidemia. Biochemical changes that occur in metabolic syndrome are very complex and include IR (not adequate response to insulin secondary to change in adipose tissues and compensatory hyperinsulinism), dyslipidemia (high LDL, TGs & ApoB and low HDL-C), chronic inflammation (high CRP, IL-6 & TNF-𝛼), Hypercoagulable state or thrombosis (high PAI-1, fibrinogen, factor-VII, factor-VIII, and low tPA), Oxidative damage (prolong oxidative and anti-oxidative impairment, high ROS, NADPH oxidase, and free radicals), adipokines dysregulation (abnormal secretion of adipokines like as glycerol, FFA and pro-inflammatory mediators) and hormonal impairment (hypercortisolism, low growth hormone secretion and hypogonadism). MS can be limited by behavior therapy (diet & exercise, lifestyle modification, and proper training), medical therapy (pharmacotherapy), and surgical procedure (BS).
References
- Halcox J, Misra A. Type 2 Diabetes Mellitus, Metabolic Syndrome, and Mixed Dyslipidemia: How Similar, How Different, and How to Treat? Metab Syndr Relat Disord. 2015;13(1):1-21. doi:10.1089/met.2014.0049 PubMed | Crossref | Google Scholar
- Foroozanfar Z, Najafipour H, Khanjani N, Bahrampour A, Ebrahimi H. The Prevalence of Metabolic Syndrome According to Different Criteria and its Associated Factors in Type 2 Diabetic Patients in Kerman, Iran. Iran J Med Sci. 2015;40(6):522-525. The Prevalence of Metabolic Syndrome According to Different Criteria and its Associated Factors in Type 2 Diabetic Patients in Kerman, Iran – PMC
- Demissie BM, Girmaw F, Amena N, Ashagrie G. Prevalence of metabolic syndrome and associated factors among patients with type 2 diabetes mellitus in Ethiopia, 2023: A systematic review and meta-analysis. BMC Public Health. 2024;24(1):1128. doi:10.1186/s12889-024-18580-0 PubMed | Crossref | Google Scholar
- Gong S, Gan S, Zhang Y, Zhou H, Zhou Q. Gamma-glutamyl transferase to high-density lipoprotein cholesterol ratio is a more powerful marker than TyG index for predicting metabolic syndrome in patients with type 2 diabetes mellitus. Front Endocrinol. 2023;14:1248614. doi:10.3389/fendo.2023.1248614 PubMed | Crossref | Google Scholar
- Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011;9(1):48. doi:10.1186/1741-7015-9-48 PubMed | Crossref | Google Scholar
- Mogre V, Salifu ZS, Abedandi R. Prevalence, components and associated demographic and lifestyle factors of the metabolic syndrome in type 2 diabetes mellitus. J Diabetes Metab Disord. 2014;13(1):80. doi:10.1186/2251-6581-13-80 PubMed | Crossref | Google Scholar
- Yao F, Bo Y, Zhao L, et al. Prevalence and Influencing Factors of Metabolic Syndrome among Adults in China from 2015 to 2017. 2021;13(12):4475. doi:10.3390/nu13124475 PubMed | Crossref | Google Scholar
- Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes Metab. 2018;20(2):427-437. doi:10.1111/dom.13088 PubMed | Crossref | Google Scholar
- Hayden MR. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. 2023;59(3):561. doi:10.3390/medicina59030561 PubMed | Crossref | Google Scholar
- Lira Neto JCG, Xavier MDA, Borges JWP, Araújo MFMD, Damasceno MMC, Freitas RWJFD. Prevalence of Metabolic Syndrome in individuals with Type 2 Diabetes Mellitus. Rev Bras Enferm. 2017;70(2):265-270. doi:10.1590/0034-7167-2016-0145 PubMed | Crossref | Google Scholar
- Ferreira JP, Verma S, Fitchett D, et al. Metabolic syndrome in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a post hoc analysis of the EMPA-REG OUTCOME trial. Cardiovasc Diabetol. 2020;19(1):200. doi:10.1186/s12933-020-01174-6 PubMed | Crossref | Google Scholar
- Nowrouzi-Sohrabi P, Hassanipour S, Sisakht M, et al. The effectiveness of pistachio on glycemic control and insulin sensitivity in patients with type 2 diabetes, prediabetes, and metabolic syndrome: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(5):1589-1595. doi:10.1016/j.dsx.2020.07.052 PubMed | Crossref | Google Scholar
- Hudish LI, Reusch JEB, Sussel L. β cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129(10):4001-4008. doi:10.1172/JCI129188 PubMed | Crossref | Google Scholar
- Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z PubMed | Crossref | Google Scholar
- Vesa CM, Zaha DC, Bungău SG. Molecular Mechanisms of Metabolic Syndrome. Int J Mol Sci. 2024;25(10):5452. doi:10.3390/ijms25105452 PubMed | Crossref | Google Scholar
- Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. 2009;120(16):1640-1645. doi:10.1161/CIRCULATIONAHA.109.192644 PubMed | Crossref | Google Scholar
- Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3-33. doi:10.1016/j.reprotox.2016.10.001 PubMed | Crossref | Google Scholar
- New SH, Leow SN, Vasudevan SK, et al. Relationship between anthropometric and biochemical changes of metabolic syndrome with retinal nerve fiber layer and macular thickness. PLoS One. 2021;16(2):e0246830. doi:10.1371/journal.pone.0246830 PubMed | Crossref | Google Scholar
- Ambroselli D, Masciulli F, Romano E, et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. 2023;15(3):640. doi:10.3390/nu15030640 PubMed | Crossref | Google Scholar
- Shiferaw WS, Akalu TY, Gedefaw M, et al. Metabolic syndrome among type 2 diabetic patients in Sub-Saharan African countries: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(5):1403-1411. doi:10.1016/j.dsx.2020.07.013 PubMed | Crossref | Google Scholar
- Regufe VMG, Pinto CMCB, Perez PMVHC. Metabolic syndrome in type 2 diabetic patients: a review of current evidence. Porto Biomed J. 2020;5(6):e101. doi:10.1097/j.pbj.0000000000000101 PubMed | Crossref | Google Scholar
- Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi:10.1155/2014/943162 PubMed | Crossref | Google Scholar
- Tribuddharatana T, Kongpiromchean Y, Sribhen K, Sribhen C. Biochemical alterations and their relationships with the metabolic syndrome components in canine obesity. Kasetsart J Nat Sci. 2011;45(4):622-628. Biochemical alterations and their relationships with the metabolic syndrome components in canine obesity
- Debnath M, Agrawal S, Agrawal A, Dubey GP. Metaflammatory responses during obesity: pathomechanism and treatment. Obes Res Clin Pract. 2016;10(2):103-113. doi:10.1016/j.orcp.2015.10.012 PubMed | Crossref | Google Scholar
- Chait A, Den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. doi:10.3389/fcvm.2020.00022 PubMed | Crossref | Google Scholar
- Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215-225. doi:10.1177/1753944717711379 PubMed | Crossref | Google Scholar
- McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36(1):14-20. doi:10.1016/j.clindermatol.2017.09.004 PubMed | Crossref | Google Scholar
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β‐cell dysfunction. Eur J Clin Invest. 2002;32(s3):14-23. doi:10.1046/j.1365-2362.32.s3.3.x PubMed | Crossref | Google Scholar
- Hayden MR, Tyagi SC. Intimal redox stress: Accelerated atherosclerosis in metabolic syndrome and type 2 diabetes mellitus. Cardiovasc Diabetol. 2002;1(1):3. doi:10.1186/1475-2840-1-3 PubMed | Crossref | Google Scholar
- Hayden MR, Tyagi SC. Is type 2 diabetes mellitus a vascular disease (atheroscleropathy) with hyperglycemia a late manifestation? Cardiovasc Diabetol. 2003;2(1):2. doi:10.1186/1475-2840-2-2 PubMed | Crossref | Google Scholar
- Hayden MR, Banks WA. Deficient leptin cellular signaling plays a key role in brain ultrastructural remodeling in obesity and type 2 diabetes mellitus. Int J Mol Sci. 2021;22(11):5427. doi:10.3390/ijms22115427 PubMed | Crossref | Google Scholar
- Dong M, Ren J. What fans the fire: Insights into mechanisms of leptin in metabolic syndrome-associated heart diseases. Curr Pharm Des. 2014;20(4):652-658. doi:10.2174/138161282004140213160930 PubMed | Crossref | Google Scholar
- Hayden MR. An immediate and long-term complication of COVID-19 may be type 2 diabetes mellitus: The central role of β-cell dysfunction, apoptosis and exploration of possible mechanisms. 2020;9(11):2475. doi:10.3390/cells9112475 PubMed | Crossref | Google Scholar
- Jabarpour M, Rashtchizadeh N, Argani H, et al. The impact of dyslipidemia and oxidative stress on vasoactive mediators in patients with renal dysfunction. Int Urol Nephrol. 2019;51(12):2235-2242. doi:10.1007/s11255-019-02319-7 PubMed | Crossref | Google Scholar
- Włodarski A, Strycharz J, Wróblewski A, et al. The role of microRNAs in metabolic syndrome-related oxidative stress. Int J Mol Sci. 2020;21(18):6902. doi:10.3390/ijms21186902 PubMed | Crossref | Google Scholar
- Razavi SM, Gholamin S, Eskandari A, et al. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J Med Food. 2013;16(3):255-258. doi:10.1089/jmf.2012.2408 PubMed | Crossref | Google Scholar
- Sun J, Luo J, Ruan Y, et al. Free fatty acids activate renin-angiotensin system in 3T3-L1 adipocytes through nuclear factor-kappa B pathway. J Diabetes Res. 2016;2016:1587594. doi:10.1155/2016/1587594 PubMed | Crossref | Google Scholar
- Yu W, Li C, Zhang D, et al. Advances in T cells based on inflammation in metabolic diseases. 2022;11(22):3554. doi:10.3390/cells11223554 PubMed | Crossref | Google Scholar
- Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: An overview. Int J Environ Res Public Health. 2020;17(20):7618. doi:10.3390/ijerph17207618 PubMed | Crossref | Google Scholar
- Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822-1832. doi:10.1038/s41591-019-0675-0 PubMed | Crossref | Google Scholar
- Lee YS, Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021;35(5-6):307-328. doi:10.1101/gad.346312.120 PubMed | Crossref | Google Scholar
- Zhang S, Gang X, Yang S, et al. The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front Immunol. 2021;12:678355. doi:10.3389/fimmu.2021.678355 PubMed | Crossref | Google Scholar
- Touch S, Clément K, André S. T cell populations and functions are altered in human obesity and type 2 diabetes. Curr Diab Rep. 2017;17(9):81. doi:10.1007/s11892-017-0900-5 PubMed | Crossref | Google Scholar
- Yi HS, Kim SY, Kim JT, et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019;10(3):249. doi:10.1038/s41419-019-1494-4 PubMed | Crossref | Google Scholar
- Xydakis AM, Case CC, Jones PH, et al. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: The impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. 2004;89(6):2697-2703. doi:10.1210/jc.2003-031 PubMed | Crossref | Google Scholar
- Guldiken S, Demir M, Arikan E, et al. The levels of circulating markers of atherosclerosis and inflammation in subjects with different degrees of body mass index: Soluble CD40 ligand and high-sensitivity C-reactive protein. Thromb Res. 2007;119(1):79-84. doi:10.1016/j.thromres.2005.12.019 PubMed | Crossref | Google Scholar
- Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89(6):2595-2600. doi:10.1210/jc.2004-0372 PubMed | Crossref | Google Scholar
- Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: Molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):H2031-H2041. doi:10.1152/ajpheart.01058.2004 PubMed | Crossref | Google Scholar
- Kazumi T, Kawaguchi A, Sakai K, Hirano T, Yoshino G. Young men with high-normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002;25(6):971-976. doi:10.2337/diacare.25.6.971 PubMed | Crossref | Google Scholar
- Yan LJ. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J Diabetes Res. 2014;2014:1-11. doi:10.1155/2014/137919 PubMed | Crossref | Google Scholar
- Fahed G, Aoun L, Bou Zerdan M, et al. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2):786. doi:10.3390/ijms23020786 PubMed | Crossref | Google Scholar
- Brookes PS. Mitochondrial H+ leak and ROS generation: An odd couple. Free Radic Biol Med. 2005;38(1):12-23. doi:10.1016/j.freeradbiomed.2004.10.016 PubMed | Crossref | Google Scholar
- Wang B, Van Veldhoven PP, Brees C, et al. Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radic Biol Med. 2013;65:882-894. doi:10.1016/j.freeradbiomed.2013.08.173 PubMed | Crossref | Google Scholar
- Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders – A step towards mitochondria-based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1066-1077. doi:10.1016/j.bbadis.2016.11.010 PubMed | Crossref | Google Scholar
- Burkart AM, Tan K, Warren L, et al. Insulin resistance in human iPS cells reduces mitochondrial size and function. Sci Rep. 2016;6(1):22788. doi:10.1038/srep22788 PubMed | Crossref | Google Scholar
- Trayhurn P. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2022;127(2):161-164. doi:10.1017/S0007114521003962 PubMed | Crossref | Google Scholar
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129-139. doi:10.1016/j.mce.2009.08.018 PubMed | Crossref | Google Scholar
- Grant RW, Dixit VD. Adipose tissue as an immunological organ. 2015;23(3):512-518. doi:10.1002/oby.21003 PubMed | Crossref | Google Scholar
- Wróblewski A, Strycharz J, Świderska E, et al. Molecular insight into the interaction between epigenetics and leptin in metabolic disorders. 2019;11(8):1872. doi:10.3390/nu11081872 PubMed | Crossref | Google Scholar
- Adamczak M, Wiecek A. The adipose tissue as an endocrine organ. Semin Nephrol. 2013;33(1):2-13. doi:10.1016/j.semnephrol.2012.12.008 PubMed | Crossref | Google Scholar
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67(1):259-284. doi:10.1146/annurev.physiol.67.040403.120816 PubMed | Crossref | Google Scholar
- Andrew R, Gale C, Walker B, Seckl J, Martyn C. Glucocorticoid metabolism and metabolic syndrome: associations in an elderly cohort. Exp Clin Endocrinol Diabetes. 2002;110(06):284-290. doi:10.1055/s-2002-34591 PubMed | Crossref | Google Scholar
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374-381. doi:10.1038/nrendo.2009.106 PubMed | Crossref | Google Scholar
- Li X, Zhai Y, Zhao J, et al. Impact of metabolic syndrome and its components on prognosis in patients with cardiovascular diseases: A meta-analysis. Front Cardiovasc Med. 2021;8:704145. doi:10.3389/fcvm.2021.704145 PubMed | Crossref | Google Scholar
- Salib A, Cayabyab F, Yoshihara E. Stem cell-derived islets for type 2 diabetes. Int J Mol Sci. 2022;23(9):5099. Published 2022 May 4. doi:10.3390/ijms23095099 PubMed | Crossref | Google Scholar
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Supplement_1):S15-33. doi:10.2337/dc21-S002 PubMed | Crossref | Google Scholar
- Poggiogalle E, Lubrano C, Sergi G, et al. Sarcopenic obesity and metabolic syndrome in adult Caucasian subjects. J Nutr Health Aging. 2016;20(9):958-963. doi:10.1007/s12603-015-0638-1 PubMed | Crossref | Google Scholar
- Gaines J, Vgontzas AN, Fernandez-Mendoza J, Bixler EO. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med Rev. 2018;42:211-219. doi:10.1016/j.smrv.2018.08.009 PubMed | Crossref | Google Scholar
- Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: International evaluation of the evidence. Eur J Cancer Prev. 2002;11 Suppl 2:S94-S100. Weight control and physical activity in cancer prevention: international evaluation of the evidence
- Uzunlulu M, Telci Caklili O, Oguz A. Association between metabolic syndrome and cancer. Ann Nutr Metab. 2016;68(3):173-179. doi:10.1159/000443743 PubMed | Crossref | Google Scholar
- Pujia R, Tarsitano MG, Arturi F, et al. Advances in Phenotyping Obesity and in Its Dietary and Pharmacological Treatment: A Narrative Review. Front Nutr.2022;9:804719. doi:10.3389/fnut.2022.804719 PubMed | Crossref | Google Scholar
- Frank AP, De Souza Santos R, Palmer BF, Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 2019;60(10):1710-1719. doi:10.1194/jlr.R086975 PubMed | Crossref | Google Scholar
- Skurk T, Hauner H. Obesity and impaired fibrinolysis: role of adipose production of plasminogen activator inhibitor-1. Int J Obes. 2004;28(11):1357-1364. doi:10.1038/sj.ijo.0802778 PubMed | Crossref | Google Scholar
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J.2018;39(33):3021-3104. doi:10.1093/eurheartj/ehy339 PubMed | Crossref | Google Scholar
- Catapano AL, Reiner Ž, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias. 2011;217(1):3-46. doi:10.1016/j.atherosclerosis.2011.06.028 PubMed | Crossref | Google Scholar
- Atar D, Jukema JW, Molemans B, et al. New cardiovascular prevention guidelines: How to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? 2021;319:51-61. doi:10.1016/j.atherosclerosis.2020.12.013 PubMed | Crossref | Google Scholar
- Gordon Smith A. Impaired glucose tolerance and metabolic syndrome in idiopathic neuropathy. J Peripheral Nervous Sys.2012;17(s2):15-21. doi:10.1111/j.1529-8027.2012.00390.x PubMed | Crossref | Google Scholar
- Pal K, Mukadam N, Petersen I, Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2018;53(11):1149-1160. doi:10.1007/s00127-018-1581-3 PubMed | Crossref | Google Scholar
- Fabricatore AN. Behavior Therapy and Cognitive-Behavioral Therapy of Obesity: Is There a Difference? J Am Diet Assoc.2007;107(1):92-99. doi:10.1016/j.jada.2006.10.005 PubMed | Crossref | Google Scholar
- Wilson PWF, Grundy SM. The Metabolic Syndrome: Practical Guide to Origins and Treatment: Part I. 2003;108(12):1422-1424. doi:10.1161/01.CIR.0000089505.34741.E5 PubMed | Crossref | Google Scholar
- El Ansari W, Elhag W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps-a Scoping Review. Obes Surg. 2021;31(4):1755-1766. doi:10.1007/s11695-020-05160-5 PubMed | Crossref | Google Scholar
Acknowledgments
The author acknowledges the endocrinology department, The First Bethune Hospital of Jilin University, Changchun, China, for the infrastructure needed to prepare this review and apologizes to researchers whose work was not cited due to space limitations.
Funding
Not reported
Author Information
Corresponding Author:
Rajiv Kumar Yadav
Department of Endocrinology and Metabolism
First Affiliated Hospital of Jilin University, China
Email: rajivakumar1@yahoo.com
Co-Authors:
Zhang Jizhou
Department of Biochemistry
College of Basic Medical Sciences, Jilin University, China
Xiaokun Gang
Department of Endocrinology and Metabolism
First Affiliated Hospital of Jilin University, China
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
Not applicable
Conflict of Interest Statement
Not reported
Guarantor
None
DOI
Cite this Article
Rajiv KY, Zhang J, Xiaokun G. Biochemical Changes in Metabolic Syndrome. medtigo J Med. 2024;2(4):e30622446. doi:10.63096/medtigo30622446 Crossref









