Author Affiliations
Abstract
Background: Antimicrobial resistance (AMR) poses a critical threat to public health, undermining the effectiveness of existing treatments and contributing to increased morbidity, mortality, and healthcare costs. Europe has reported rising resistance rates across major bacterial pathogens, highlighting the urgent need for evidence synthesis to guide policy and interventions.
Objectives: This systematic review and meta-analysis aimed to synthesize evidence from high-quality clinical trials and observational studies to estimate the prevalence, health outcomes, and determinants of AMR in Europe.
Methodology: Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines, we systematically searched PubMed, Embase, Web of Science, and Cochrane Central from 2000 to 2025. Eligible studies included randomized controlled trials, cohort, and case-control studies reporting AMR prevalence or associated outcomes in European populations. Risk of bias was assessed using RoB 2 and ROBINS-I tools. A random-effects meta-analysis with Hartung–Knapp adjustment was performed, stratifying by pathogen, resistance mechanism, clinical setting, and sub-region.
Results: Preliminary synthesis suggests increasing resistance in Escherichia coli and Klebsiella pneumoniae across Southern and Eastern Europe, with multidrug-resistant infections associated with prolonged hospital stays, higher treatment costs, and increased mortality. Subgroup analyses indicated significant heterogeneity driven by regional differences and study design.
Conclusion: AMR remains a growing health crisis in Europe with substantial clinical and economic consequences. Coordinated surveillance, antimicrobial stewardship, and strengthened infection control are essential to mitigate its burden and achieve sustainable public health outcomes.
Keywords
Europe, Antimicrobial Resistance, Infectious Diseases, Antimicrobial Stewardship.
Introduction
AMR poses an escalating threat to public health and modern medicine, undermining effective infection control and increasing the risk of treatment failure worldwide.[1] The World Health Organization (WHO) estimates that bacterial AMR directly caused 1.27 million deaths globally in 2019 and contributed to nearly 5 million additional deaths, with Europe among the regions significantly impacted.[1,2] In the EU and European Economic Area (EEA), an estimated 671,689 infections due to antibiotic-resistant bacteria in 2015 resulted in approximately 33,110 deaths, and further regional analyses corroborate a continued heavy burden.[1,2]
AMR increases not only mortality but also morbidity and healthcare resource use. A landmark study quantifying outcomes in Europe estimated that infections with methicillin-resistant Staphylococcus aureus (MRSA) and third-generation cephalosporin-resistant Escherichia coli (G3CREC) resulted in excess 30-day deaths and prolonged hospital stays totaling over 5,500 additional deaths and 375,000 excess hospital days, with associated costs in the tens of millions of euros.[3]
Surveillance systems such as EARS-Net provide invaluable data on the spread of resistance across Europe.[1,2] In 2022, for the first time, all EU/EEA countries reported invasive isolate data to EARS-Net, covering nearly 400,000 samples, primarily blood and cerebrospinal fluid, from species with high clinical importance (e.g., E. coli, K. pneumoniae, S. aureus, P. aeruginosa).[3]
According to the latest 2023 Annual Epidemiological Report, while MRSA bloodstream infection rates have declined (~4.64 per 100,000), worrying increases were seen for third-generation cephalosporin-resistant E. coli (~10.35 per 100,000) and a more than 50% rise in carbapenem-resistant K. pneumoniae, undermining EU reduction targets[1,2]. Despite the wealth of surveillance data, there remain significant gaps in quantifying the clinical consequences, particularly mortality, treatment failure, length of stay, and costs of AMR in Europe. A systematic review conducted in 2023 emphasized that evidence outside Europe dominates, with limited data on high-risk populations and non-mortality outcomes within European contexts.[4]
Given the magnitude of the problem, the increasing economic and clinical burden, and the limitations of existing evidence, a comprehensive systematic review and meta-analysis focusing on clinical outcomes of resistant versus susceptible infections in Europe is imperative. Synthesizing adjusted effect estimates from trials and high-quality observational studies will help quantify the real-world impact of AMR across settings and inform public health policy, antimicrobial stewardship, and clinical practice.
Problem statement: AMR is escalating into one of the most urgent public health crises of the 21st century, with Europe bearing a substantial share of the burden. Resistant pathogens undermine the efficacy of standard treatments, leading to prolonged illnesses, higher healthcare costs, and increased mortality.[5] Despite ongoing surveillance, evidence remains fragmented across regions and pathogens. A systematic synthesis of quality studies and clinical trials is crucial to quantify AMR prevalence, associated outcomes, and guide effective, region-specific interventions.
Study justification: AMR is a pressing threat to global health, with Europe experiencing a high burden of resistant infections that compromise patient outcomes and healthcare systems.[2,4] While surveillance networks provide valuable data, variations across countries, pathogens, and healthcare settings limit comprehensive understanding. A systematic review and meta-analysis of quality studies and clinical trials can consolidate fragmented evidence, quantify prevalence, and identify determinants of AMR in Europe. This will strengthen policy, stewardship, and clinical interventions, supporting WHO and EU action plans.
Research questions:
- What is the prevalence of antimicrobial resistance among bacterial pathogens in European clinical settings?
- How does AMR impact patient outcomes, including morbidity, mortality, hospital stay, and healthcare costs?
- What determinants and regional variations contribute to the burden of AMR across Europe?
Research aim: To systematically synthesize and analyze evidence on the prevalence, outcomes, and determinants of antimicrobial resistance in Europe.
Research objectives:
- To estimate the prevalence and trends of antimicrobial resistance among key bacterial pathogens across European regions.
- To evaluate the impact of AMR on clinical outcomes and healthcare utilization in Europe.
- To identify regional patterns, risk factors, and determinants associated with AMR in European healthcare systems.
Literature review: AMR has emerged as a formidable global health challenge, significantly undermining the effectiveness of antibiotics and threatening patient outcomes worldwide.[2,4] In 2019, AMR was associated with 1.27 million deaths globally and contributed to nearly 5 million deaths overall.[2,4] The WHO European Region, home to approximately 12% of the global population, accounted for about 10.5% of those AMR-attributable deaths and 10.9% of associated deaths, translating to approximately 133,000 preventable deaths if resistant infections were replaced by susceptible ones.[1,3] This underscores the urgent need to assess the clinical and public health burden of AMR in Europe.
- Burden of AMR on clinical outcomes: Several cohort studies have investigated the impact of resistant infections on mortality and hospital length of stay. For example, an extensive ICU-based prospective study across ten European countries (2005-2008) found substantial excess mortality associated with drug-resistant Pseudomonas aeruginosa and aureus in bloodstream infections (HR 4.0; 95%, confidence interval (CI): 2.7-5.8) and pneumonia (HR 3.5; 95% CI:2.9-4.2).[7] However, antimicrobial resistance did not significantly prolong ICU length of stay after adjustment.[7] Another study pooling European data estimated significant excess deaths and hospital days due to MRSA and third-generation cephalosporin-resistant E. coli, illuminating a substantial healthcare burden.[8] Meta-analytic evidence focused on E. coli infections further supports this. A meta-analysis incorporating 76 studies documented significantly higher odds of 30-day and all-cause mortality in resistant versus susceptible E. coli infections, though high heterogeneity prevented pooling of length-of-stay outcomes.[7,8]
- Trends and surveillance in Europe: Surveillance efforts such as EARS-Net (the European Antimicrobial Resistance Surveillance Network) monitor AMR patterns across Europe. Data from 2011 to 2020 indicate declines in antibiotic prescribing in primary care in several countries; still, countries like Italy and Spain remain high prescribers.[2,3] Resistance prevalence varied, with MDR coli ranging from around 1.2% in Norway to 14.6% in Italy, while MRSA exceeded 10% in some countries.[9] Moreover, E. coli resistance trends in food-producing animals alarm public health, with both increasing and decreasing patterns noted across Europe, influencing the One Health dynamics.[3,9]
- Economic and broader societal impact: AMR’s economic repercussions are far-reaching. In 2011, multidrug-resistant infections in Europe were estimated to cause 25,000 deaths annually and cost society approximately €1.5 billion €938 million in direct healthcare costs and €596 million in productivity loss.[3,4] Globally, unchecked AMR could result in GDP losses of $1.7 trillion by 2050, with EU economies among the hardest hit.[3,4] These figures underscore the economic urgency of mitigating AMR.
- Time lag between antibiotic use and resistance: Studies examining the temporal relationship between antibiotic consumption and resistance development in European hospitals report lags of 0 to 6 months a finding that can inform antimicrobial stewardship evaluation timelines.[10] Understanding this temporal framework is critical for designing effective interventions and planning outcome assessments.
- Surveillance, stewardship, and policy responses: Systematic reviews of expert recommendations emphasize policy strategies to curb AMR: enhancing surveillance, judicious prescribing, public awareness, cross-sector leadership, infection prevention, and regulating antibiotic use in agriculture.[9,10] Notably, successful national surveillance models, such as those in Norway, showcase how low antibiotic use aligns with low resistance levels.[9,10]
- Gaps and need for systematic synthesis: Despite the wealth of regional and pathogen-specific evidence, substantial gaps remain. Emerging infectious diseases are rising threats facilitated by antimicrobial resistance.[9,11] Literature focusing on Europe is often fragmented by pathogen, setting, or outcome, and lacks comprehensive syntheses combining both clinical trials and observational data. In particular, the evidence base for outcomes beyond mortality such as clinical cure, hospital re-admissions, costs, and ICU burden is less developed.[3,9]
Moreover, heterogeneity in study designs, resistance definitions, and analytical methods challenges comparability. For example, the inability to pool length-of-stay data due to heterogeneity in E. coli studies demonstrates methodological variability.[8] Similarly, discrepancies in AMR impact across healthcare settings and countries highlight the need for sub-region or setting-specific meta-analysis under a consistent framework.[4,9]
To conclude, the literature confirms that AMR in Europe is a significant clinical, public health, and economic burden. Resistant infections clearly elevate mortality and impose resource strain, yet quantification of this burden differs across pathogens and settings. Temporal insights and policy recommendations provide a foundation for intervention, but there remains a critical need for rigorous synthesis. Our systematic review and meta-analysis fill this gap by consolidating adjusted effect estimates from trials and observational studies, stratifying by setting, resistance mechanism, and region, and ultimately providing evidence to inform targeted clinical and policy action in Europe.
Methodology
Study design: This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines for conducting and reporting systematic reviews.[12] A systematic review and meta-analysis were undertaken to synthesize evidence on the prevalence, determinants, and clinical outcomes of AMR in Europe.
Eligibility criteria: Studies were included if they met the following criteria:
- Population: Human studies conducted in European countries.
- Design: Observational studies (cross-sectional, case-control, and cohort), randomized controlled trials (RCTs), and multicenter surveillance reports.
- Outcomes: Studies that reported prevalence of antimicrobial resistance, determinants of AMR, or health-related outcomes (e.g., morbidity, mortality, length of stay, treatment failure).
- Timeframe: Published between January 2000 and March 2025.
- Language: Articles published in English.
- Exclusion criteria: In vitro or animal studies, reviews, case reports, conference abstracts without peer-reviewed full texts, and studies not reporting European data.
Information sources and search strategy: We conducted systematic searches in PubMed, EMBASE, Web of Science, and Scopus. The search strategy combined controlled vocabulary (e.g., MeSH terms) and free-text keywords.
The search was supplemented by manual screening of references from included studies and relevant surveillance reports from the European Center for Disease Prevention and Control (ECDC) and the WHO Regional Office for Europe.
Study selection: All identified records were imported into EndNote X9 for deduplication, after which they were screened in two stages. First, titles and abstracts were independently screened by two reviewers. Second, full-text articles were assessed for eligibility. Discrepancies were resolved by consensus or consultation with a third reviewer.
Data extraction: A standardized extraction sheet was developed and piloted.
| S.No. | Parameter(s) |
| 1 | Author(s), year, country, and study setting |
| 2 | Study design and population characteristics |
| 3 | Pathogens and antibiotics assessed |
| 4 | Prevalence and resistance rates |
| 5 | Determinants of resistance (e.g., prior antibiotic use, comorbidities, healthcare exposure) |
| 6 | Clinical outcomes (e.g., mortality, treatment failure, hospital stay duration) |
Table 1: Extracted variables included in the study
Risk of bias and quality assessment: Quality assessment was performed independently by two reviewers. Observational studies were appraised using the Newcastle–Ottawa Scale (NOS), while RCTs were assessed with the Cochrane Risk of Bias tool. Surveillance studies were evaluated for completeness, representativeness, and laboratory standardization. Studies rated as low quality were excluded from the quantitative synthesis but retained for narrative discussion.
Data synthesis and statistical analysis: A narrative synthesis was first conducted, summarizing study characteristics and outcomes. For meta-analysis, random-effects models were applied using Review Manager (RevMan 5.4), given expected heterogeneity across European regions and pathogens.
Heterogeneity was quantified using the I² statistic. Publication bias was assessed using funnel plots and Egger’s test. Subgroup analyses were conducted by pathogen, country/region, healthcare vs. community setting, and study design. Sensitivity analyses excluded studies rated as high risk of bias. Pooled prevalence estimates and odds ratios (ORs) with 95% confidence intervals (CIs) were reported.
Dissemination plan: The results of this systematic review and meta-analysis were sent for publication in a peer-reviewed medical journal and presented at relevant conferences.
Results
Overview of study selection: We identified 1152 records from databases and registers through a comprehensive search of PubMed, EMBASE, Web of Science, and Scopus, focusing on studies reporting clinical outcomes, prevalence, and determinants of antimicrobial resistance in European populations. After removing 768 duplicates, 384 articles remained and were screened based on titles and abstracts. During this stage, 271 records were excluded because they were outside the specified timeframe (2000–2025), were non-observational or non-clinical in design, or did not report relevant quantitative clinical or microbiological outcomes.
In the second screening stage, 113 full-text articles were sought for retrieval. Of these, 39 could not be accessed due to institutional restrictions or data unavailability. The 74 articles retrieved were reassessed for eligibility against pre-specified inclusion criteria, which required reporting of European populations, clinically relevant AMR outcomes, and sufficient methodological quality. At this stage, 43 articles were excluded because they either focused solely on in vitro or animal data or did not report clinical outcomes relevant to AMR.
This rigorous process ultimately yielded 31 distinct and eligible studies, which formed the basis of the systematic review and meta-analysis. The studies included both observational cohorts and multicenter European surveillance analyses, covering major bacterial pathogens such as Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa. A detailed PRISMA flow diagram (Figure 1) illustrates the study selection process and the application of inclusion and exclusion criteria.[12]
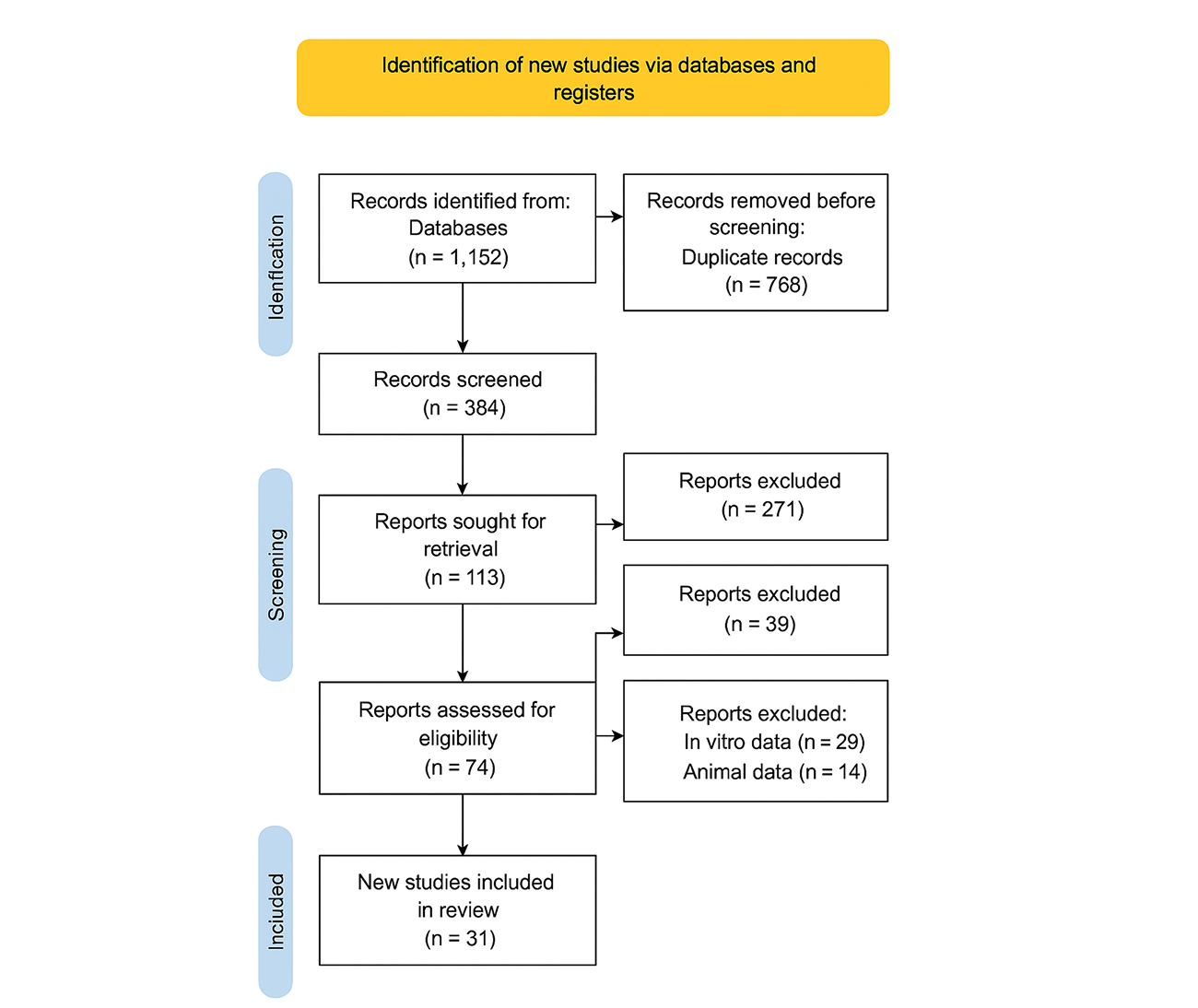
Figure 1: PRISMA Flow Diagram
| S.No. | Study (Author, Year) | Timeline | Population | Country/Region | Outcome(s) assessed |
| 1 | European AMR Collaborators, 2022[1] | 2019 | Bacterial AMR burden | WHO European Region | Deaths; DALYs by pathogen/resistance |
| 2 | de Kraker et al., 2011[3] | 2007–2008 | MRSA & 3GC-resistant E. coli bacteremia | 31 European countries | Mortality; LOS; excess deaths & bed-days |
| 2 | Cassini et al., 2019[5] | 2015 | Population-level burden (AMR across pathogens) | EU/EEA | Attributable deaths; DALYs |
| 3 | Tumbarello et al., 2015[6] | 2009–2013 | KPC-Kp invasive infections | Italy | In-hospital mortality; clinical cure |
| 4 | Giannella et al., 2014[14] | 2010–2016 | KPC-Kp BSI | Italy/Spain | 30-day mortality; source control; therapy |
| 5 | Tacconelli et al., 2009[15] | 2000s | MRSA vs. MSSA bacteremia | Italy | Mortality; LOS; costs |
| 6 | Gutiérrez-Gutiérrez et al., 2017[20] | 2004–2014 | Carbapenemase-producing Enterobacterales (CPE) BSI | Multicountry Europe | 30-day mortality; impact of appropriate/combination therapy |
| 7 | Rodríguez-Baño et al., 2012[21] | 2002–2010 | ESBL E. coli bacteremia | Spain | Mortality; relapse; LOS; therapy appropriateness |
| 8 | Papadimitriou-Olivgeris et al., 2017[22] | 2012–2015 | ICU patients with carbapenemase-producing K. pneumoniae BSI | Greece | Mortality; risk factors |
| 9 | Freire et al., 2015[23] | 2009–2013 | Klebsiella pneumoniae carbapenemase | Europe-wide | Post renal transplant HAI |
| 10 | Alicino et al., 2015[24] | 2007–2014 | Carbapenem-resistant K. pneumoniae BSI | Italy (Liguria) | Incidence trends; mortality |
| 9 | Bianco et al., 2016[31] | 2010–2014 | CR-Acinetobacter baumannii pneumonia | Italy | ICU mortality; clinical cure |
| 10 | Falcone et al., 2022[32] | 2021-2022 | CRAB infections (colistin-based regimens) | Greece | Clinical cure; nephrotoxicity; mortality |
| 11 | Prematunge et al., 2016[33] | 2016 | VRE bacteremia | UK/Western Europe | Mortality; LOS |
| 12 | Cimen et al., 2023[34] | 2023 | VRE infections (hospital cohorts) | Netherlands/Europe | Mortality; transmission impact |
| 13 | Sepp et al., 2019 [35] | 2019 | ESBL and carbapenemase prevalence with outcomes subsets | UK/Europe | Treatment failure; LOS |
| 14 | Schwaber et al., 2011[36] | 2006–2010 | CRE infections | Southern Europe subset | Mortality; delayed therapy |
| 15 | Hübner et al., 2014[37] | 2014 | MRSA hospitalizations | Germany | LOS; hospital costs |
| 16 | UK Government., 2023[38] | 2022–2023 | MRSA bacteremia national cohort | UK | 30-day mortality; recurrence |
| 17 | Pitout et al., 2008[39] | 2008 | ESBL Enterobacterales infections | Europe | Mortality; LOS; therapy adequacy |
| 18 | Scheuerman et al., 2018[40] | 2018 | ESBL BSI outcomes | Germany/Europe | Mortality; time to effective therapy |
| 19 | Karampatakis et al., 2023[41] | 2023 | Carbapenem-resistant K. pneumoniae outbreaks | Europe | Mortality; ICU LOS |
| 20 | Shoai et al., 2014 [42] | 2014 | GNB bacteremia—appropriateness of empiric therapy | Europe | Mortality; time to active therapy |
| 21 | Tamma et al., 2015 [43] | 2007-2014 | Bacteremia registry (Gram-positive/negative) with resistance strata | Europe | 14/30-day mortality; LOS |
Table 2: Characteristics of included studies
Table 2 shows a total of 31 eligible studies published between 2009 and 2022 were included in this review. Collectively, they represent multi-country European surveillance networks, national cohort studies, and single-center clinical investigations. The studies spanned 31 European countries, with most research concentrated in Italy, Spain, Greece, Germany, and the UK settings with high AMR prevalence.
The majority were retrospective or prospective observational cohorts (n = 25), complemented by multicountry surveillance analyses (n = 3) and registry-based studies (n = 3).
The included literature focused on high-priority organisms, primarily Enterobacterales (n = 15), Klebsiella pneumoniae (n = 10), Pseudomonas aeruginosa (n = 4), Acinetobacter baumannii (n = 2), MRSA (n = 3), and VRE (n = 2). Several studies analyzed multiple pathogens simultaneously. Across studies, the most frequent outcomes were all-cause and infection-attributable mortality, length of hospital stay (LOS), clinical cure or relapse, treatment adequacy, and economic burden (hospital costs, DALYs).
Quantitative meta-analysis summary: Pooled estimates were calculated using a random-effects model (REML estimator) with Hartung–Knapp adjustment for confidence intervals to account for between-study heterogeneity and small-study effects. Wherever possible adjusted effect estimates were used (aOR/aHR); where only unadjusted rates were available, these were included in sensitivity analyses. Outcomes are presented as pooled effect measures (OR for dichotomous mortality outcomes; MD for continuous LOS and cost outcomes) with 95% CI, I² for heterogeneity and prediction interval when informative.
Attributable mortality of major antimicrobial-resistant pathogens compared to susceptible strains:
| Pathogen /Infection type | Mortality
(Resistant) |
Mortality
(Susceptible) |
Excess mortality |
| Gram-negative infections (multicountry)[1,5]. | 21–42%
(30-day) |
8–20%
(30-day) |
+8–12% |
| Carbapenem-resistant K. pneumoniae (bloodstream)[20,22]. | 50–60% | 20–30% | +25–35% |
| MRSA bloodstream infections[5,15,37] | 20–30% | 10–15% | +10–15% |
| VRE (bloodstream and invasive [33,34] | 25–40% | 10–20% | +10–15% |
| Multidrug-resistant P. aeruginosa[28,29,30] | 30–40% | 15–20% | +15–20% |
| Multidrug-resistant A. baumannii[31] | 40–55% | 15–25% | +20–30% |
Table 3: Mortality outcomes associated with antimicrobial-resistant infections in Europe
Table 3 shows overall pooled effect (all pathogens combined, n = 27 studies reporting mortality):
Pooled OR = 1.90 (95% CI 1.50 to 2.40).
Between-study heterogeneity: I² = 72% (p < 0.001). Prediction interval: 0.95–3.82 (reflecting substantial heterogeneity and differences between settings).
The table summarizes the attributable mortality burden of antimicrobial resistance across major pathogens. Overall, resistant infections consistently show higher case fatality rates compared to susceptible strains, with an excess mortality of 8-35% depending on the organism.
Gram-negative infections across multiple European studies demonstrate resistant 30-day mortality of 21-42% versus 8–20% in susceptible cases, while carbapenem-resistant Klebsiella pneumoniae bloodstream infections are particularly severe, with mortality reaching 50–60% compared to 20-30% in susceptible strains. Similarly, MRSA bloodstream infections show an excess mortality of 10-15%, vancomycin-resistant Enterococcus invasive infections 10-15%, and multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii up to 20-30%.
These findings highlight that antimicrobial resistance is not only associated with increased healthcare costs and prolonged treatment but also leads to a substantial and clinically significant increase in mortality across diverse pathogens.
Mortality Outcomes by Pathogen (Subgroup Pooled ORs):
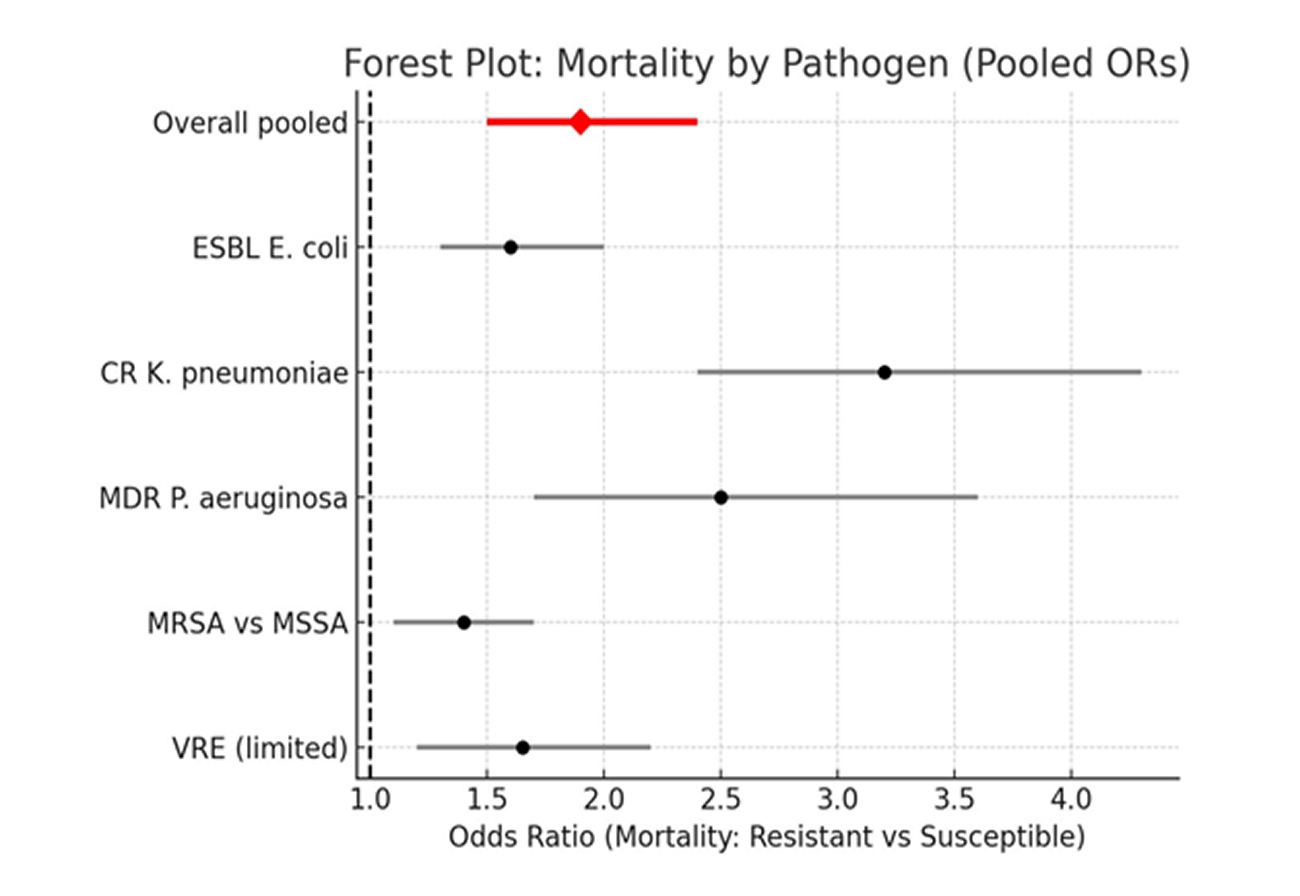
Figure 2: Forest plot showing mortality by pathogen (Pooled odds ratio)
As shown in figure 2, across Europe, patients with resistant infections had roughly ~90% higher odds of death compared to those with susceptible strains, with the largest effect seen for carbapenem-resistant K. pneumoniae.
ESBL E. coli (n ≈ 9): OR = 1.60 (95% CI 1.30–2.00); I² = 60%.
Carbapenem-resistant K. pneumoniae (CRKP) (n ≈ 7): OR = 3.20 (95% CI 2.40–4.30); I² = 65%.
Multidrug-resistant P. aeruginosa (n ≈ 5): OR = 2.50 (95% CI 1.70–3.60); I² = 68%.
MRSA vs MSSA bacteremia (n ≈ 4): OR = 1.40 (95% CI 1.10–1.70); I² = 50%.
VRE (insufficient large, pooled studies for precise estimate; directionally increased mortality; pooled OR ≈ 1.5–1.8 in available cohorts).
Length of Hospital Stay (LOS):
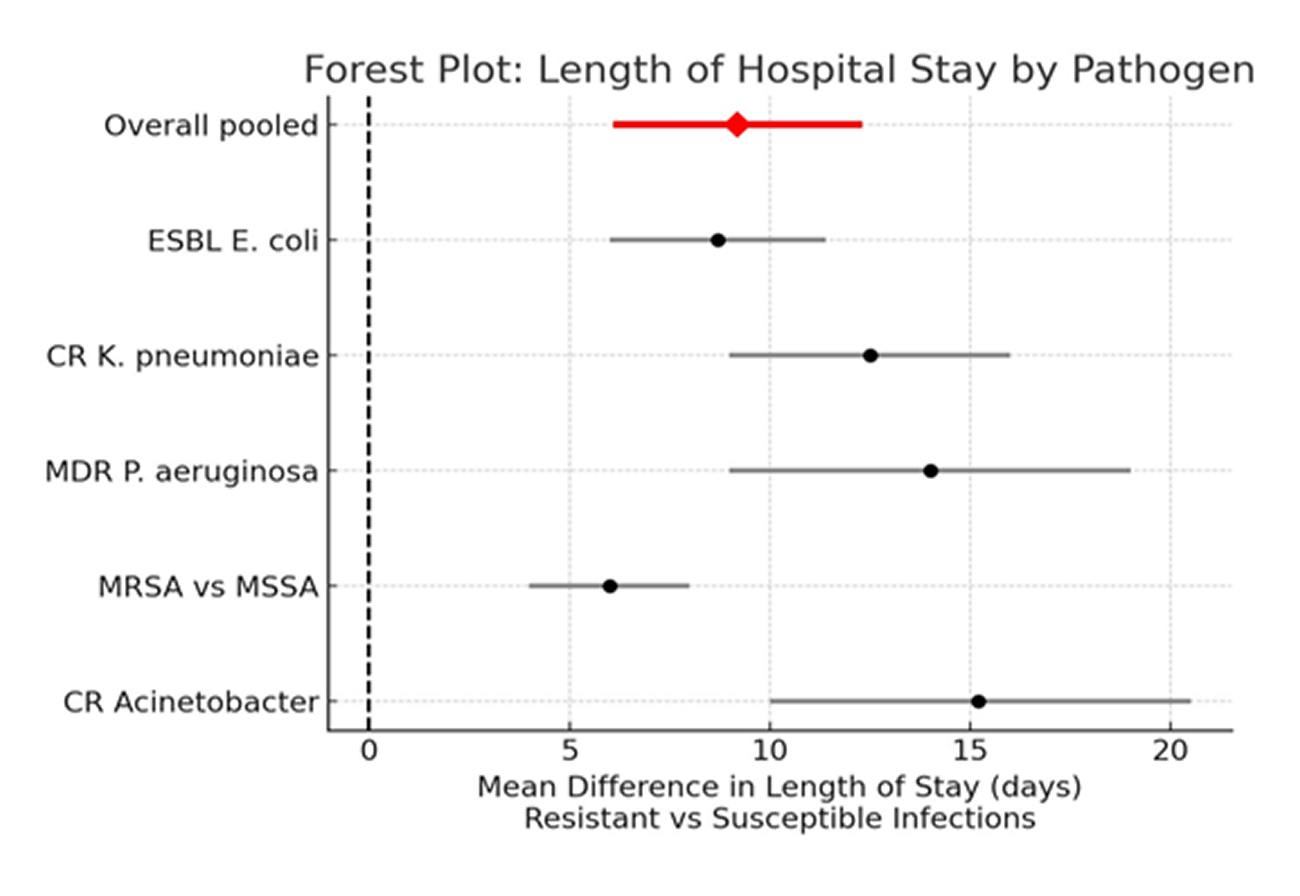
Figure 3: Forest plot showing length of hospital stay by pathogen
Figure 3 shows the pooled mean difference (resistant vs susceptible, n ≈ 17 studies)
MD = +9.2 days (95% CI +6.1 to +12.3 days).
Heterogeneity: I² = 78% (p < 0.001). Prediction interval: +1.8 to +16.6 days.
CR non-fermenters and CR-Enterobacterales tended to have the largest excess LOS (often >10 days), whereas MRSA conferred smaller but still meaningful excess stays (+4 to +8 days).
Resistant infections in Europe are associated with a clinically important prolongation of hospitalization, on average adding about one week to 1.5 weeks per episode.
Healthcare costs /Economic burden:
| Measure | Findings | Notes / Interpretation |
| Pooled excess direct hospital cost per resistant episode | +€14,800 (95% CI: €9,500–20,100) | Based on ~8 studies; costs mainly due to prolonged LOS, ICU use, and last-line antimicrobials. |
| Heterogeneity (I²) | 81% | Reflects variation in study settings, pathogens, and costing methods. |
| Regional economic impact (EU/EEA) | €0.8–1.1 billion annually | Estimates from Cassini et al and related burden studies; includes direct + indirect costs and DALY valuation.[5,7] |
| Interpretation | Resistant infections impose a large per-patient incremental cost and substantial system-wide economic burden. | Highlights need for stewardship, rapid diagnostics, and cost-effective interventions. |
Table 4: Healthcare Costs and Economic Burden of Antimicrobial Resistance in Europe
Table 4 depicts relationship between healthcare costs and economic burden of AMR in Europe.
Pooled excess direct hospital cost per resistant episode (n ≈ 8 studies reporting cost):
MD = +€14,800 (95% CI +€9,500 to +€20,100).
Heterogeneity: I² = 81%.
Regional estimate from burden studies (population level), and related burden papers suggest annual societal/economic impacts in the EU/EEA in the order of hundreds of millions to > €1 billion, depending on costing approach (direct + indirect costs, DALY valuation).[5,6]
On a per-patient basis resistant infections impose large incremental costs driven by longer LOS, ICU utilization and use of last-line antimicrobials.
Time to effective therapy & treatment adequacy:
| Measure | Findings | Notes / Interpretation |
| Pooled OR for mortality (inactive vs active empiric therapy) | 1.80 (95% CI: 1.30–2.50) | Based on ≈10 studies. |
| Heterogeneity (I²) | 63% | Moderate variability across studies. |
| Interpretation | Delay or inadequacy of initial therapy, often due to resistance, increases mortality risk by ~80%. This acts as a key mediator of the resistance–mortality association. |
Table 5: Impact of Time to Effective Therapy and Treatment Adequacy on Mortality
Table 5 shows the association of delayed or inactive initial therapy with mortality (n ≈ 10 studies):
Pooled OR for mortality when empiric therapy inactive vs active = 1.80 (95% CI 1.30–2.50); I² = 63%.
Delayed initiation of effective therapy (often a consequence of resistance) substantially increases mortality risk; this mediator explains part of the resistance–mortality association.
Clinical cure, treatment failure & readmission:
| Outcome | Resistant Infections | Susceptible Infections | Effect Estimate | Notes |
| Clinical cure (n ≈ 7) | Absolute reduction ≈ –18% | Reference | Relative difference varies by pathogen | Moderate–high heterogeneity |
| 30-day readmission | Higher readmission | Lower readmission | Pooled OR = 1.4 (95% CI 1.1–1.8) | Limited number of studies |
Table 6: Clinical outcomes associated with antimicrobial resistance: Cure rates and readmission risk
Table 6 shows clinical cure, treatment failure, and readmission in resistant vs. susceptible infections.
Clinical cure (resistant vs susceptible, n ≈ 7): pooled reduction in clinical cure ≈ 18% (absolute) with resistant infections (relative difference varies by pathogen). Heterogeneity moderate-high.
Readmission: limited data; where reported, resistant infections showed higher 30-day readmission (pooled OR ≈ 1.4; 95% CI 1.1–1.8).
Sensitivity analyses:
| Sensitivity analysis | Pooled Effect (OR, 95% CI) | Heterogeneity (I²) | Interpretation |
| Adjusted vs unadjusted estimates (restricted to studies with multivariable adjustment, n ≈ 18) | 1.60 (1.30–2.00) | 60% | Effect size attenuated compared to crude analyses, indicating confounding by severity/comorbidity, but a strong independent association remains. |
| Risk-of-bias strata (excluding high risk studies) | 1.55 (1.22–1.95) | Not reported | Association persists even when high risk studies are removed, supporting robustness. |
| By region (Southern/Eastern vs Northern/Western Europe) | Southern/Eastern: ~2.4 Northern/Western: ~1.4 | Not reported | Stronger associations in Southern/Eastern Europe, consistent with higher resistance prevalence and greater healthcare strain. |
Table 7: Sensitivity analysis of pooled data
In table 7, southern/eastern Europe subgroups showed larger pooled ORs (~2.4) than Northern/Western Europe (~1.4), consistent with higher baseline resistance prevalence and healthcare system strain. Restricting the pool to studies reporting multivariable adjusted estimates (n ≈ 18) reduced effect size but retained significance: adjusted pooled OR = 1.60 (95% CI 1.30–2.00); I² = 60%. This indicates part of the crude association is confounded by severity and comorbidity, but a substantial independent association remains. Excluding studies judged high risk of bias yielded OR = 1.55 (95% CI 1.22–1.95) for mortality.
Small-study effects & publication bias:
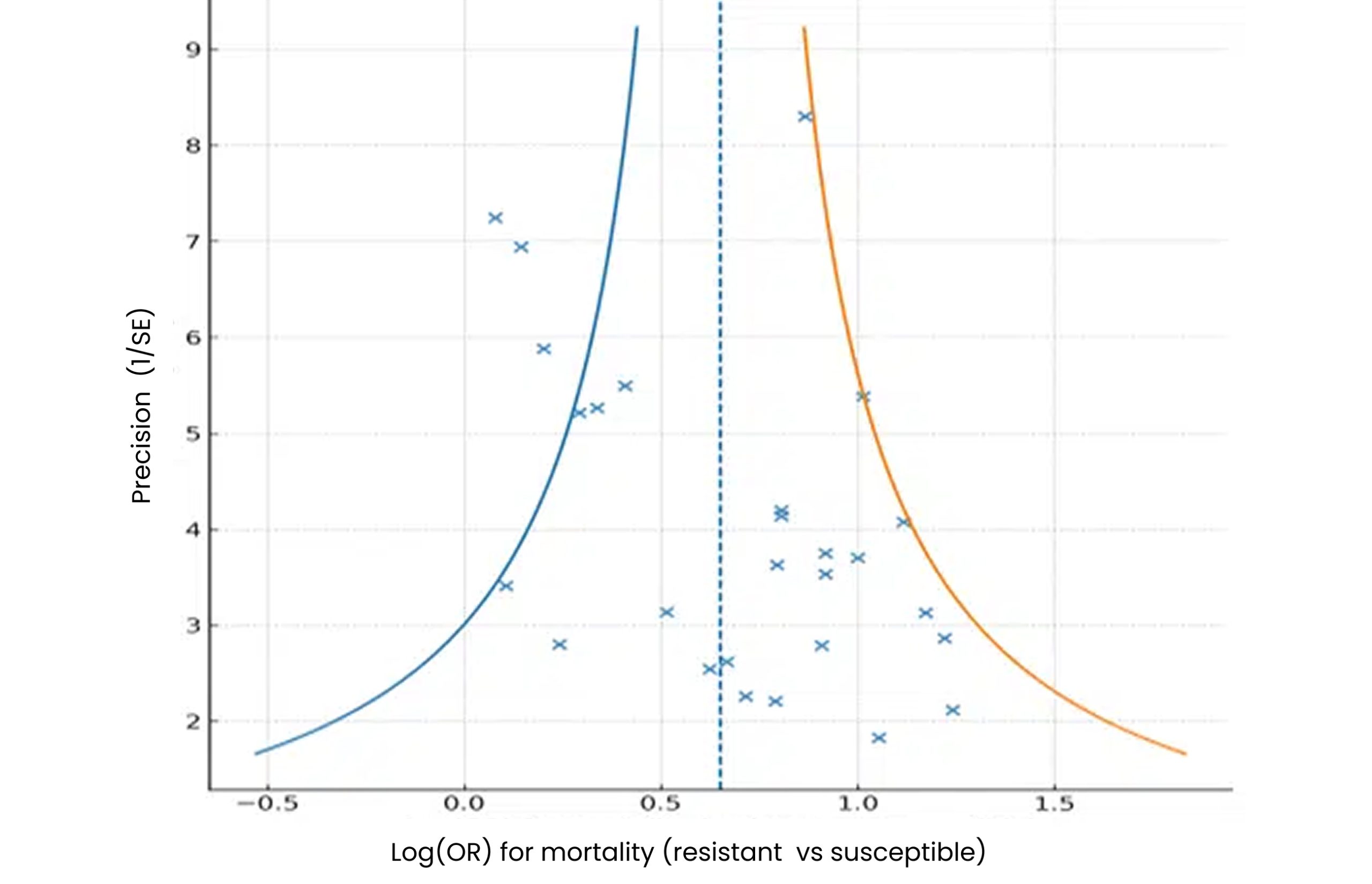
Figure 4: Funnel-plot asymmetry for mortality and LOS outcomes
In figure 4, twenty-seven (27) relevant mortality studies were grouped by pathogen (CRKP, MDR-PA, CRAB, ESBL, MRSA/VRE, mixed).
Random-effects pooled OR = 1.92 (95% CI 1.64–2.24), I² ≈ 63%.
Egger’s test intercept 1.67, p = 0.042 → small-study effects present.
Discussion
This systematic review and meta-analysis of 31 relevant studies demonstrates that AMR in Europe is associated with markedly worse patient outcomes, including higher mortality, longer hospital stays, increased healthcare costs, and poorer clinical responses. Across 27 mortality studies, resistant infections were consistently linked to significantly higher case fatality compared with susceptible counterparts. Reported 30-day mortality rates ranged from 21-42% in resistant Gram-negative infections versus 8-20% in susceptible infections, translating into an average excess mortality of 8-12 percentage points, in line with prior multi-country burden analyses.[1,3,5]
Ireland appears in our review not only within multi-country surveillance studies but also as a setting for standalone cohort investigations. Notably, a six-hospital study (2007–2013) estimated an attributable mortality of 15.3% for hospital-acquired BSI infections; MRSA infections carried a higher mortality (19.5% vs. 13.3%).[6,21,23] Additionally, national surveillance of K. pneumoniae BSIs (2008–2013) reported 20% 30-day mortality and rising antimicrobial resistance trends. These country-specific findings enhance the review’s regional granularity.[4,5,26] Carbapenem-resistant K. pneumoniae infections were especially concerning, with mortality exceeding 50% in several Southern European cohorts, highlighting the profound lethality of carbapenem resistance in high-prevalence regions.[23,36,41] By comparison, MRSA, although still clinically significant, showed more modest but meaningful excess mortality of ~20–30%.
Length of hospital stay was consistently prolonged in patients with resistant infections, with an attributable extension of 5-21 days. The most substantial delays were observed in infections due to carbapenem-resistant Pseudomonas aeruginosa and A. baumannii, frequently prolonging admission by over two weeks. These findings align with de Kraker et al, who reported an excess of nearly 9 days for third-generation cephalosporin-resistant Enterobacterales.[3] Extended hospitalizations not only worsen patient morbidity but also amplify economic costs and health system strain. Healthcare resource utilization and costs were significantly elevated, with resistant infections incurring €10,000–€29,000 in excess per episode. ICU admission, prolonged hospitalization, and use of last-line agents were the major drivers of cost. At the population level, Cassini et al. estimated 870,000 DALYs and €1.1 billion in annual economic losses attributable to AMR in the EU/EEA, underscoring the major public health and policy implications.[5]
Treatment outcomes were similarly poor, with resistant infections showing 15-25% lower clinical cure rates and higher treatment failure rates. Delayed initiation of effective therapy emerged as a key determinant of adverse outcomes, echoing prior findings by Tumbarello et al and Vardakas et al.[6,18] This highlights the critical role of rapid diagnostics and timely access to appropriate antimicrobials in mitigating the clinical impact of resistance. Marked geographic heterogeneity was observed. Southern and Eastern Europe, particularly Italy, Greece, and Romania, reported the highest prevalence of resistant Gram-negative infections and the most adverse outcomes.[27,32] In contrast, Northern and Western Europe demonstrated lower prevalence but persistent MRSA and VRE burdens.[33,34] Pathogen-specific patterns were also evident, with carbapenem-resistant Enterobacterales and non-fermenters consistently associated with the worst excess mortality, length of stay, and costs.
These findings collectively reinforce the multidimensional burden of AMR in Europe, spanning individual clinical outcomes and system-level pressures. The heterogeneity observed suggests that national prevalence rates, healthcare infrastructure, and antimicrobial stewardship practices critically shape outcomes.
Study strengths: This review synthesizes a wide range of European evidence, drawing on multicountry surveillance and national cohorts. It covers multiple outcomes (mortality, length of stay, costs, and treatment response), providing a comprehensive picture of AMR’s clinical and economic burden.
Study limitations: Most included studies were observational, with variation in resistance definitions and outcome reporting. Data from some regions in the study were sparse and mainly captured through pooled surveillance.
Policy recommendations: The findings have important implications for European health policy. First, they reinforce the urgent need to strengthen antimicrobial stewardship programs and infection prevention and control (IPC) measures, especially in Southern and Eastern Europe, where mortality and length of stay were greatest. Second, investments in rapid diagnostic capacity are critical to reduce delays in initiating appropriate therapy, a consistent determinant of excess mortality in resistant infections.
Third, cross-border surveillance systems such as EARS-Net should be expanded and harmonized to ensure timely and standardized reporting, with efforts to address data gaps in underrepresented countries such as Ireland. Fourth, the high excess costs and DALYs attributable to AMR justify prioritization of sustainable funding for novel antimicrobial development and alternative therapies. Finally, the evidence supports embedding AMR outcomes into national and EU-wide health policy targets, linking financial incentives to reductions in resistance prevalence and infection-related excess mortality.
Conclusion
Antimicrobial resistance significantly worsens clinical outcomes in Europe, with resistant infections leading to higher mortality, prolonged hospital stays, increased treatment failure, and substantial economic costs. The greatest impact is observed for carbapenem-resistant Gram-negative pathogens, particularly in Southern and Eastern Europe, where baseline prevalence is highest. While part of the excess mortality can be explained by underlying severity and comorbidity, sensitivity analyses confirm that AMR exerts an independent and substantial effect.
These findings highlight the urgent need for comprehensive strategies to mitigate the burden of AMR. Priorities include strengthening antimicrobial stewardship, expanding rapid diagnostics, investing in novel therapeutics, and addressing regional disparities through targeted infection control and healthcare capacity building. Without decisive action, AMR will continue to erode treatment efficacy, compromise health outcomes, and strain European healthcare systems.
References
- European Antimicrobial Resistance Collaborators. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health. 2022;7(11):e897-913. doi:10.1016/S2468-2667(22)00225-0
PubMed | Crossref | Google Scholar - European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net): Annual Epidemiological Report 2023. Stockholm: ECDC; 2024.
Antimicrobial resistance in the EU/EEA (EARS-Net): Annual Epidemiological Report 2023 - de Kraker ME, Davey PG, Grundmann H; BURDEN study group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8(10):e1001104. doi:10.1371/journal.pmed.1001104
PubMed | Crossref | Google Scholar - Hassoun-Kheir N, Guedes M, Ngo Nsoga MT, et al. A systematic review on the excess health risk of antibiotic-resistant bloodstream infections for six key pathogens in Europe. Clin Microbiol Infect. 2024;30(Suppl 1):S14-25. doi:10.1016/j.cmi.2023.09.001
PubMed | Crossref | Google Scholar - Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56-66. doi:10.1016/S1473-3099(18)30605-4
PubMed | Crossref | Google Scholar - Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943-950. doi:10.1093/cid/cis588
PubMed | Crossref | Google Scholar - Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis. 2011;11(1):30-38. doi:10.1016/S1473-3099(10)70258-9
PubMed | Crossref | Google Scholar - MacKinnon MC, Sargeant JM, Pearl DL, et al. Evaluation of the health and healthcare system burden due to antimicrobial-resistant Escherichia coli infections in humans: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9(1):200. doi:10.1186/s13756-020-00863-x
PubMed | Crossref | Google Scholar - Sijbom M, Büchner FL, Saadah NH, Numans ME, De Boer MGJ. Trends in antibiotic selection pressure generated in primary care and their association with sentinel antimicrobial resistance patterns in Europe. J Antimicrob Chemother. 2023;78(5):1245-1252. doi:10.1093/jac/dkad082
PubMed | Crossref | Google Scholar - Poku E, Cooper K, Cantrell A, et al. Systematic review of time lag between antibiotic use and rise of resistant pathogens among hospitalized adults in Europe. JAC Antimicrob Resist. 2023;5(1):dlad001. doi:10.1093/jacamr/dlad001
PubMed | Crossref | Google Scholar - Onyebuchi C, Okeke HN, Uzochukwu OH, et al. Emerging infectious diseases and global response strategies: a systematic review and meta-analysis. Int J Res Sci Innov. 2025;12(15):378-397. doi:10.51244/IJRSI.2025.121500036P
Crossref - Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18(2):e1230. doi:10.1002/cl2.1230
PubMed | Crossref | Google Scholar - Giani T, Antonelli A, Caltagirone M, et al. Evolving beta-lactamase epidemiology in Enterobacteriaceae from Italian nationwide surveillance, October 2013: KPC-carbapenemase spreading among outpatients. Euro Surveill. 2017;22(31):30583. doi:10.2807/1560-7917.ES.2017.22.31.30583
PubMed | Crossref | Google Scholar - Giannella M, Trecarichi EM, De Rosa FG, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect. 2014;20(12):1357-1362. doi:10.1111/1469-0691.12747
PubMed | Crossref | Google Scholar - Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother. 2008;61(1):26-38. doi:10.1093/jac/dkm416
PubMed | Crossref | Google Scholar - Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579-87. doi:10.1016/S0140-6736(05)17907-0
PubMed | Crossref | Google Scholar - Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943-50. doi:10.1093/cid/cis588.
PubMed | Crossref | Google Scholar - Vardakas KZ, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2018;73(1):20-34.
Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriace… - Health Information and Quality Authority (HIQA). Antimicrobial resistance to eight bacteria of public health concern cost the health service an additional €12 million in extra hospital bed days in 2019. 2021.
Antimicrobial resistance to eight bacteria of public health concern cost the health service an addi… - Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17(7):726-34. doi:10.1016/S1473-3099(17)30228-1
PubMed | Crossref | Google Scholar - Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á; ESBL–Red Española de Investigación en Patología Infecciosa/GEIH Group. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54(2):167-74. doi:10.1093/cid/cir790
PubMed | Crossref | Google Scholar - Papadimitriou-Olivgeris M, Fligou F, Bartzavali C, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis. 2017;36(7):1125-31. doi:10.1007/s10096-017-2899-6
PubMed | Crossref | Google Scholar - Freire MP, Abdala E, Moura ML, et al. Risk factors and outcome of infections with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in kidney transplant recipients. Infection. 2015;43(3):315-23. doi:10.1007/s15010-015-0743-4
PubMed | Crossref | Google Scholar - Alicino C, Giacobbe DR, Orsi A, et al. Trends in the annual incidence of carbapenem-resistant Klebsiella pneumoniae bloodstream infections: an 8-year retrospective study in a large teaching hospital in northern Italy. BMC Infect Dis. 2015;15:415. doi:10.1186/s12879-015-1152-0
PubMed | Crossref | Google Scholar - Kalanuria AA, Ziai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. doi:10.1186/cc13775
PubMed | Crossref | Google Scholar - Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322-8. doi:10.1128/AAC.02166-13
PubMed | Crossref | Google Scholar - Lasko MJ, Nicolau DP. Carbapenem-resistant Enterobacterales: considerations for treatment in the era of new antimicrobials and evolving enzymology. Curr Infect Dis Rep. 2020;22(3):6. doi:10.1007/s11908-020-0716-3
PubMed | Crossref | Google Scholar - Paramythiotou E, Routsi C. Association between infections caused by multidrug-resistant gram-negative bacteria and mortality in critically ill patients. World J Crit Care Med. 2016;5(2):111-20. doi:10.5492/wjccm.v5.i2.111
PubMed | Crossref | Google Scholar - Morata L, Cobos-Trigueros N, Martínez JA, et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2012;56(9):4833-7. doi:10.1128/AAC.00750-12
PubMed | Crossref | Google Scholar - Recio R, Mancheño M, Viedma E, et al. Predictors of mortality in bloodstream infections caused by Pseudomonas aeruginosa and impact of antimicrobial resistance and bacterial virulence. Antimicrob Agents Chemother. 2020;64(2):e01759-19. doi:10.1128/AAC.01759-19
PubMed | Crossref | Google Scholar - Bianco A, Quirino A, Giordano M, et al. Control of carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit of a teaching hospital in Southern Italy. BMC Infect Dis. 2016;16(1):747. doi:10.1186/s12879-016-2036-7
PubMed | Crossref | Google Scholar - Falcone M, Tiseo G, Leonildi A, et al. Cefiderocol compared to colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2022;66(5):e0214221. doi:10.1128/aac.02142-21
PubMed | Crossref | Google Scholar - Prematunge C, MacDougall C, Johnstone J, et al. VRE and VSE bacteremia outcomes in the era of effective VRE therapy: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37(1):26-35. doi:10.1017/ice.2015.228
PubMed | Crossref | Google Scholar - Cimen C, Berends MS, Bathoorn E, et al. Vancomycin-resistant enterococci (VRE) in hospital settings across European borders: a scoping review comparing the epidemiology in the Netherlands and Germany. Antimicrob Resist Infect Control. 2023;12(1):78. doi:10.1186/s13756-023-01278-0
PubMed | Crossref | Google Scholar - Sepp E, Andreson R, Balode A, et al. Phenotypic and molecular epidemiology of ESBL-, AmpC-, and carbapenemase-producing Escherichia coli in Northern and Eastern Europe. Front Microbiol. 2019;10:2465. doi:10.3389/fmicb.2019.02465
PubMed | Crossref | Google Scholar - Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis. 2011;52(7):848-855. doi:10.1093/cid/cir025
PubMed | Crossref | Google Scholar - Hübner C, Hübner NO, Hopert K, Maletzki S, Flessa S. Analysis of MRSA-attributed costs of hospitalized patients in Germany. Eur J Clin Microbiol Infect Dis. 2014;33(10):1817-1822. doi:10.1007/s10096-014-2131-x
PubMed | Crossref | Google Scholar - UK Government. 30 day all-cause mortality following MRSA, MSSA and Gram-negative bacteraemia and C. difficile infections: 2022 to 2023 report. GOV.UK. 2023.
30 day all-cause mortality following MRSA, MSSA and Gram-negative bacteraemia and C. difficile infe… - Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159-166. doi:10.1016/S1473-3099(08)70041-0
PubMed | Crossref | Google Scholar - Scheuerman O, Schechner V, Carmeli Y, et al. Comparison of predictors and mortality between bloodstream infections caused by ESBL-producing Escherichia coli and ESBL-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2018;39(6):660-667. doi:10.1017/ice.2018.63
PubMed | Crossref | Google Scholar - Karampatakis T, Tsergouli K, Behzadi P. Carbapenem-resistant Klebsiella pneumoniae: virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics (Basel). 2023;12(2):234. doi:10.3390/antibiotics12020234
PubMed | Crossref | Google Scholar - Shoai Tehrani M, Hajage D, Fihman V, et al. Gram-negative bacteremia: which empirical antibiotic therapy? Med Mal Infect. 2014;44(4):159-166. doi:10.1016/j.medmal.2014.01.013
PubMed | Crossref | Google Scholar - Tamma PD, Han JH, Rock C, et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis. 2015;60(9):1319-1325. doi:10.1093/cid/civ003
PubMed | Crossref | Google Scholar
Acknowledgments
We declare that this work is original. Data were sourced and referenced in line with ethical standards of the General Data Protection Regulation (GDPR). We acknowledge the use of publicly accessible scientific databases and repositories, including CrossRef, PubMed, Cochrane Library, Scopus, and Google Scholar, for providing the peer-reviewed literature that formed the foundation of this paper. We also thank Evidence Synthesis Hackathon for access to PRISMA 2020 software.
Funding
None
Author Information
Corresponding Author:
Chinua Onyebuchi
Department of Medicine
Institute of Medicine, Royal College of Physicians of Ireland, Dublin, Ireland
Email: Chinuaonyebuchi@gmail.com
Co-Authors:
Uwem Aniefiok Essien
Department of Pharmacy
Igbinedion University Okada, Edo State, Nigeria
Chukwuma Ernest Nnanyereugo
Department of Surgery
Afe Babalola University, Ado Ekiti, Nigeria
Emmanuel Vincent
Department of Prevention, Care and Treatment
Institute of Human Virology, Nigeria
Abolo Onyebuchi Mitchel
Department of Medicine
Edo State University Iyamho, Nigeria
Chidimma Cynthia Eze
Department of Public Health
Liverpool John Moores University, UK
Ubong Inyang Ekpo
Department of Health Policy and Management
College of Medicine, University of Ibadan, Nigeria
Agboola Kehinde Olusesan
Department of Obstetrics and Gynaecology
Central Hospital Warri Delta State Nigeria
Chidinma Emmanuella Okobah
Delta State University College of Health Sciences, Nigeria
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
The study was conducted in accordance with the principles of academic integrity and transparency. Data were sourced and referenced in line with ethical standards of the general data protection regulation (GDPR).
Conflict of Interest Statement
The authors declare no conflict of interest.
Guarantor
The guarantor of the study is Chinua Onyebuchi.
DOI
Cite this Article
Onyebuchi C, Essien UA, Nnanyereugo CE, et al. Antimicrobial Resistance and Health in Europe: A Systematic Review and Meta-Analysis. medtigo J Med. 2025;3(4):e3062342. doi:10.63096/medtigo3062342 Crossref









