Author Affiliations
Abstract
A 61-year-old male with a history of cerebrovascular accident (CVA) and stage 3 hypertension presented with drowsiness and abdominal pain. The initial treatment included ondansetron, telmisartan, pantoprazole, aspirin, amlodipine, and Hosit. Adjustments were made after the patient’s condition worsened, including the addition of atorvastatin, clopidogrel, and promethazine. However, the patient developed gastrointestinal bleeding, prompting the discontinuation of clopidogrel and the administration of low molecular-weight heparin (LMWH). The patient’s condition improved after these adjustments, and he was discharged. The Naranjo Adverse drug reaction (ADR) probability scale and drug interaction probability scale (DIPS) were used to assess the likelihood of drug-induced adverse reactions and interactions. The Naranjo score of 7 indicated a probable ADR, while DIPS suggested a likely drug-drug interaction (DDI). This case highlights the complexities of managing multiple medications in a patient with CVA and hypertension.
Keywords
Cerebrovascular accident, Adverse drug reactions, Drug-drug interaction, Naranjo scale hypertension, Clopidogrel.
Introduction
A usual dosage of 75 mg of clopidogrel is linked to an increased risk of gastrointestinal bleeding, which can show up as blood in the stool. With hazard ratios of 3.66 and 3.52, respectively, clopidogrel has been shown to substantially increase the risk of upper gastrointestinal bleeding (UGIB) and lower gastrointestinal bleeding (LGIB).[1] A study found that 12% of high-risk individuals had a history of gastrointestinal bleeding or peptic ulcer illness, indicating that these patients are more at risk.[2] This risk is further increased by variables including advanced age, chronic kidney disease, and concomitant use of drugs like non-steroidal anti-inflammatory drugs (NSAIDs) or warfarin.[3-5] As a result, even while clopidogrel successfully reduces thrombotic events, patients and medical professionals need to keep an eye out for symptoms of gastrointestinal bleeding, such as blood in the stool.[2]
The majority of ADRs were classified as “probable” by the Naranjo causality scale and World health organization-Uppsala monitoring centre (WHO-UMC) causality evaluation scale, boosting confidence in the diagnostic accuracy.[6] Because they can result in side effects and decreased therapeutic efficacy, DDIs are important factors to consider when using pharmacotherapy. Pharmacodynamic, pharmacokinetic, and pharmacological interactions are the three primary categories into which these interactions can be divided.[7] While pharmacokinetic interactions entail modifications in the absorption, distribution, metabolism, or elimination of medications because of their combined use, pharmacodynamic interactions take place at the site of drug action.[8] Given the complexity of drug combinations in clinical settings, it is imperative to identify potential DDIs. This can be effectively accomplished by utilizing machine learning models that forecast not only the presence of interactions but also their types and severities. Moreover, medical personnel frequently lack sufficient understanding of DDIs, emphasizing the necessity of better awareness and education to promote patient safety.
Case Presentation
A 61-year-old man presented with a history of drowsiness for the last two days, but with unknown allergies, and abdominal pain. He has a medical history of CVA and stage 3 hypertension. The patient was initially prescribed the following medications: Emeset (ondansetron) 4 mg as needed, telmisartan 40 mg once daily (OD), and an injection. Additionally, they were given Pan (pantoprazole) 40 mg, Escospirin (aspirin) 150 mg OD, and amlodipine 2.5 mg OD. The following day, the medications were adjusted to include Storvas (atorvastatin) 20 mg OD, Cervin (clopidogrel) 75 mg OD, Promolet 25 mg OD, and telmisartan 40 mg was continued.
After three days, the patient’s blood pressure stabilized, but they developed blood in the stools and worsening drowsiness. As a result, clopidogrel (Cervin) 75 mg was withdrawn, and LMWH (Clexane) 40 mg OD subcutaneously was started. A magnetic resonance imaging (MRI) showed no new CVA. Following these changes, the patient’s blood in stools resolved, but they experienced insomnia, leading to the discontinuation of Escospirin (aspirin) 150 mg OD. After these adjustments, the patient’s condition improved significantly, and they were discharged.
Case Management
The primary goals of this case’s management were to stabilize the patient’s hypertension and stop additional CVA episodes, but consequences like drowsiness and gastrointestinal bleeding necessitated careful changes. The bleeding problem was lessened when LMWH was substituted for clopidogrel. Another factor in the patient’s recovery from insomnia was quitting aspirin. In subsequent follow-ups, careful monitoring of blood pressure and possible adverse effects from drugs such as telmisartan, atorvastatin, and Clexane will be required to guarantee the patient’s stability. DDIs are measured using Medscape, LaxiCamp software, and ADRs are measured using the Naranjo assessment scale. At a tertiary care hospital, data were gathered with an emphasis on follow-up cases.
Naranjo Score: Figure 2 and Table 2 show that an ADR was likely based on the Naranjo ADR probability scale score of 7.
Drug interaction probability: The improvement in the patient’s health after stopping clopidogrel was evidence of a positive interaction recorded by the DIPS following the co-administration of clopidogrel and aspirin. The likelihood of a DDI was indicated by the DIPS score.
Clinical observations: The patient’s sleepiness and gastrointestinal bleeding stopped after stopping clopidogrel and modifying other drugs. After stopping the aspirin, the patient’s insomnia, which had started after the medication was given, went away, and they were discharged.
Discussion
Using the Naranjo ADR probability scale, we can assess the probability that clopidogrel caused the patient’s blood in stools by going over the scale’s questions and allocating scores appropriately. This case serves as an example of the difficulties in managing polypharmacy in a patient who has several comorbidities, including hypertension and CVA. Following the prescription of clopidogrel and aspirin, which are frequently prescribed for secondary prevention in CVA but are known to increase the risk of gastrointestinal side effects, the patient experienced severe sleepiness and gastrointestinal bleeding. Figure 1 and Table 1 show that ADR is probable in our situation since the Naranjo score was determined to be 1 + 2 + 1 + 0 + 2 + 1 + 0 = 7 (that is probable). The patient’s gastrointestinal bleeding was most likely caused by clopidogrel and aspirin, according to the Naranjo ADR probability scale, which was used to assess the likelihood of the adverse event (score of 7).
| Naranjo’s score | |||||
| S.No | Question | Sure | Not sure | No idea | Score in our case |
| 1 | Does this response have any prior conclusive reports? | 1 | 0 | 0 | 1 |
| 2 | Did the suspected medication cause the adverse event | 2 | -1 | 0 | 2 |
| 3 | When the medication was stopped or a particular antagonist was given, did the adverse reaction get better? | 1 | 0 | 0 | 1 |
| 4 | When the medication was given again, did the adverse reaction manifest? | +2 | 1 | 0 | 0 |
| 5 | Could the reaction have been brought on by any other factors except the drug? | -1 | +2 | 0 | +2 |
| 6 | Was there a hazardous concentration of the medication found in any bodily fluids? | +1 | 0 | 0 | 0 |
| 7 | When a placebo was administered, did the reaction resurface? | -1 | +1 | 0 | 0 |
| 8 | Did a higher dose cause a more severe reaction, or did a lower amount cause a less severe reaction? | +1 | 0 | 0 | 0 |
| 9 | Has the patient already experienced a similar reaction to the same or comparable drugs? | +1 | 0 | 0 | 0 |
| 10 | Did objective evidence support the detrimental events? | +1 | 0 | 0 | 1 |
Table 1: Score scale for Naranjo surveys
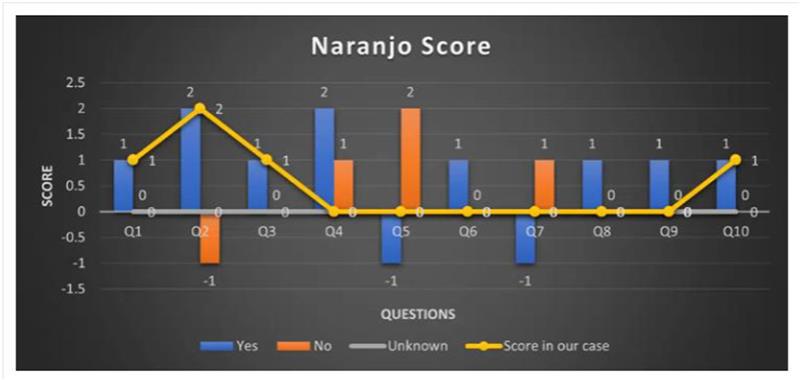
Figure 1: Naranjo ADR probability score
| S.No | DIPS questions | Answer | Result |
| 1 | Are there previous reports of the interaction? | Yes | +1 |
| 2 | Did the interaction occur after co-administration of the drugs? | Yes |
+2 |
| 3 | Did the adverse effect improve after discontinuation of one of the drugs? | Yes | +1 |
| 4 | Could other factors have caused the interaction (e.g., disease)? | Yes | +1 |
| 5 | Was the interaction more severe when the dose of one or both drugs was increased? | No | 0 |
| 6 | Was the interaction confirmed by objective evidence (e.g., blood levels)? | No | 0 |
| 7 | Did the adverse event reappear when the drugs were re-administered? | No | 0 |
Table 2: DIPS question score
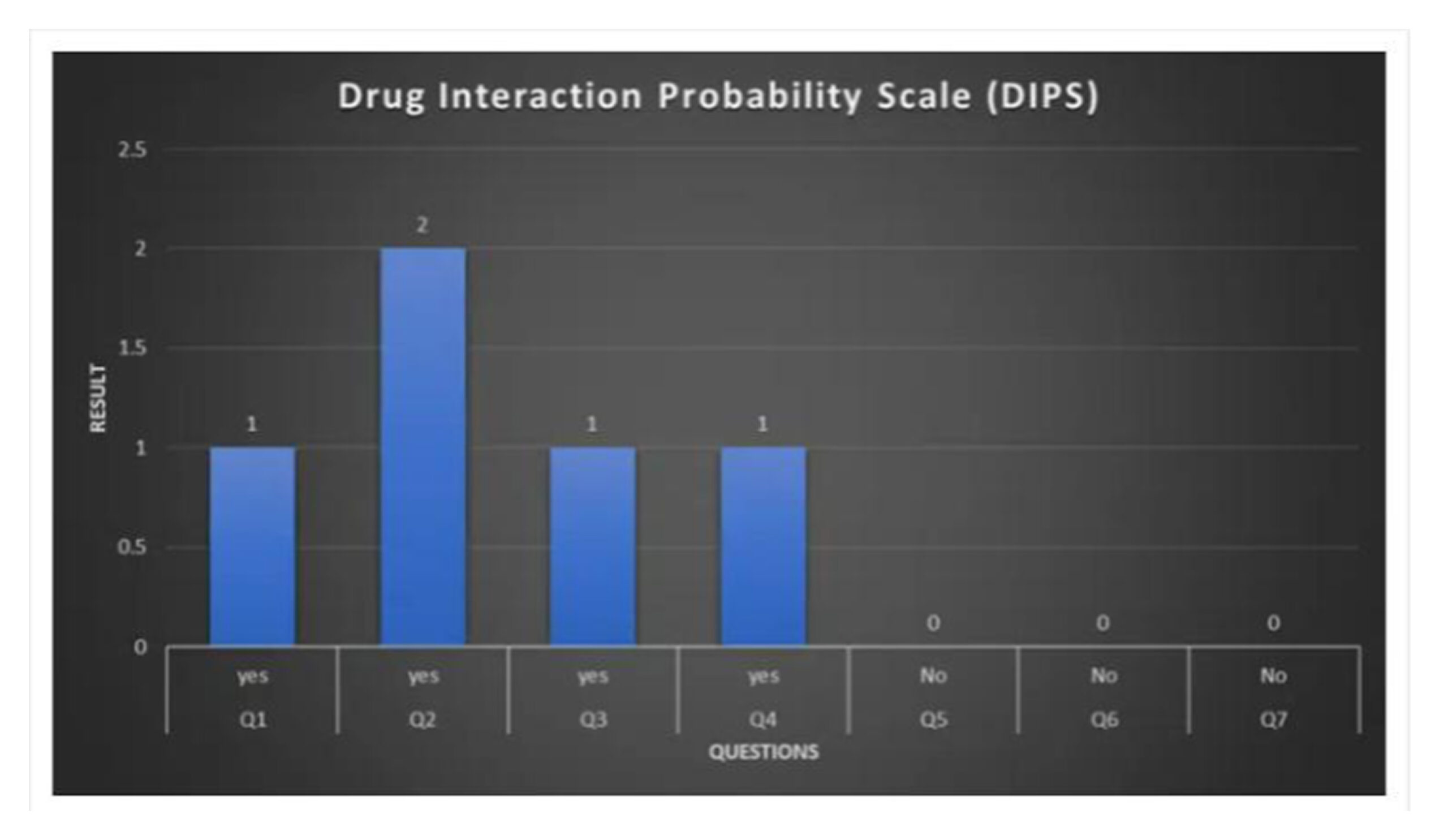
Figure 2: DIPS Score
The adverse event was most likely caused by a DDI between clopidogrel and aspirin, according to the DIPS. By stopping clopidogrel and aspirin, the interaction was lessened, which eventually resulted in the patient’s release and the cessation of the gastrointestinal bleeding.
Conclusion
The Naranjo ADR Probability Scale and DIPS were used to successfully identify and manage the patient’s ADR and DDI. The patient’s symptoms of insomnia and gastrointestinal bleeding improved after stopping clopidogrel and aspirin. The significance of careful ADR and DDI evaluation in complex individuals with concomitant conditions like hypertension and CVA is demonstrated by this example. Patient outcomes can be enhanced, and major complications can be avoided with proper medication management.
References
- Ng FH, Wong SY, Chang CM, Chen WH, Kng C, Lanas AI, Wong BCY. High incidence of clopidogrel-associated gastrointestinal bleeding in patients with previous peptic ulcer disease. Aliment Pharmacol Ther. 2003;18(4):443-449. doi:10.1046/j.1365-2036.2003.01693.x PubMed | Crossref | Google Scholar
- Bjorkman DJ. Clopidogrel Increases the Risk for Gastrointestinal Bleeding. NEJM journal watch. 2013. Clopidogrel Increases the Risk for Gastrointestinal Bleeding
- Nguyen KA, Eadon MT, Yoo R, et al. Risk Factors for Bleeding and Clinical Ineffectiveness Associated with Clopidogrel Therapy: A Comprehensive Meta-Analysis. Clin Transl Sci. 2021;14(2):645-655. doi:10.1111/cts.12926 PubMed | Crossref | Google Scholar
- Cuschieri JR, Drawz P, Falck-Ytter Y, Wong RCK. Risk factors for acute gastrointestinal bleeding following myocardial infarction in veteran patients who are prescribed clopidogrel. J Dig Dis. 2014;15(4):195-201. doi:10.1111/1751-2980.12123 PubMed | Crossref | Google Scholar
- Prasad SD, Satyender K, Rakesh KS, Amrish C, Seema, Dinesh K. Unveiling the therapeutic landscape of oseltamivir: Exploring drug utilization, adverse reactions, and interactions with comorbidities—a prospective study. Open Access Journal of Pharmaceutical Research. 2024;8(1):000301. doi:10.23880/oajpr-16000301 Crossref
- Abdul Raheem AK, Dhannoon BN. A novel deep learning model for drug-drug interactions. Curr Comput Aided Drug Des. 2024;20(5):666-672. doi:10.2174/0115734099265663230926064638 PubMed | Crossref | Google Scholar
- Ghoneim MM. Drug interaction in anaesthesia: a review. Canad Anaesth Soc J. 1971;18:353-375. doi:10.1007/BF03025688 PubMed | Crossref | Google Scholar
- Mei S, Zhang K. A machine learning framework for predicting drug-drug interactions. Sci Rep. 2021;11(1):17619. doi:10.1038/s41598-021-97193-8 PubMed | Crossref | Google Scholar
Acknowledgments
Not reported
Funding
Not reported
Author Information
Corresponding Author:
Dil Prasad Subba
Department of Pharmacy
School of Pharmacy, Sharda University, Greater Noida, Uttar Pradesh, India
Email: dilprasadsubba60@gmail.com
Co-Author:
Sharma Ravi
Department of Pharmacy
School of Pharmacy, Sharda University, Greater Noida, Uttar Pradesh, India
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation, and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Informed consent
The written informed consent for the publication of this case report was obtained from the patient, and the identification details (name and address) of the patient were kept private as per request.
Conflict of Interest Statement
Not reported
Guarantor
None
DOI
Cite this Article
Dil Prasad S, Sharma R. Adverse Drug Reactions and Drug Interaction: GI Bleeding and Insomnia in a Hypertensive CVA Patient. medtigo J Med. 2024;2(4):e30622474. doi:10.63096/medtigo30622474 Crossref









