Author Affiliations
Abstract
Background: Liver toxicity, or hepatotoxicity, arises from diverse causes including metabolic syndromes, alcohol abuse, drug-induced injury, and immune dysregulation. This review aims to explore emerging therapeutic strategies for liver toxicity stemming from diverse etiologies, including metabolic disorders, drug-induced liver injury, and alcohol-related liver disease. By analyzing clinical evidence on probiotics, N-acetylcysteine (NAC), curcumin–galactomannoside complex (CGM), and macrophage-based cell therapy, the article highlights their efficacy, mechanisms, and translational potential.
Methodology: A systematic review was conducted using PubMed and Google Scholar to identify human clinical trials, randomised controlled trials (RCTs), and observational studies published between January 2015 and June 2025. After screening 11,020 initial articles and applying rigorous inclusion and exclusion criteria, four high-quality studies were selected for final analysis.
Results: Probiotics showed subgroup-specific improvements in liver markers among patients with depressive episodes, suggesting a role for the gut-liver-brain axis. NAC significantly reduced alanine transaminase (ALT), alkaline phosphatase (ALP), and bilirubin in drug-induced liver injury, with sustained benefits and good tolerability. CGM led to pronounced improvements in liver enzymes, oxidative stress, and inflammation in alcohol-induced liver dysfunction. Lastly, infusion of alternatively activated macrophages in paracetamol-induced liver injury showed a favorable safety profile and potential regenerative effects, though efficacy data remain preliminary.
Conclusion: Targeted therapies such as NAC and CGM exhibit strong clinical potential for managing hepatotoxicity, while probiotics and macrophage therapy may benefit specific populations. Personalized, mechanism-driven approaches, validated by larger multicenter trials, are essential for improving outcomes in liver toxicity.
Keywords
Hepatotoxicity, Oxidative stress, Probiotics, N-acetylcysteine, Curcumin-galactomannoside complex, Macrophage therapy, Liver enzymes, Gut-liver axis.
Introduction
Liver toxicity, or hepatotoxicity, represents a critical and complex clinical challenge with multifactorial origins, ranging from metabolic disturbances and alcohol abuse to drug-induced damage and emerging immunological mechanisms. In recent years, a growing body of literature has underscored the increasing burden of liver injury across diverse populations, fueled by lifestyle changes, substance use, and polypharmacy.[1] A multisociety Delphi consensus has redefined steatotic liver disease (SLD) to reflect its heterogeneous etiologies, including Metabolic dysfunction-associated steatotic liver disease (MASLD), alcohol-associated liver disease (ALD), a combination known as MetALD, and forms of cryptogenic or specific-etiology liver steatosis.[2] MASLD, formerly recognized as non-alcoholic fatty liver disease (NAFLD), now affects nearly a quarter of the global population and has emerged as a silent epidemic due to its asymptomatic progression and the risk of advancing to fibrosis and cirrhosis.[3]
One of the high-risk subgroups for MASLD comprises individuals with co-existing psychiatric disorders, particularly depression. The frequent overlap of poor dietary patterns, sedentary lifestyle, substance abuse (especially alcohol), and psychotropic medications in this population increases their vulnerability to liver damage.[4] Moreover, emerging evidence highlights that beyond behavioral and metabolic risk factors, the gut-liver-brain axis plays a pivotal role in linking mood disorders and hepatic pathology. Dysbiosis, or microbial imbalance in the gut, can lead to increased intestinal permeability and translocation of microbial metabolites into the liver via the portal circulation, driving inflammation, oxidative stress, and hepatocyte injury.[5] This mechanistic pathway not only contributes to liver damage but may also exacerbate depressive symptoms, suggesting a bidirectional relationship.[6]
To explore therapeutic strategies that might benefit both hepatic and psychiatric outcomes, probiotics have garnered interest due to their ability to modulate gut microbiota and reduce systemic inflammation.[7] In patients with depressive disorders, probiotics have been investigated for their potential to alleviate psychiatric symptoms and attenuate hepatic steatosis and fibrosis. However, existing trials yield mixed results, highlighting the need to identify specific patient characteristics that might predict treatment efficacy.[8]
Another widely studied therapeutic in the realm of hepatotoxicity is N-acetylcysteine (NAC). Best known for its role in treating paracetamol-induced liver injury, NAC functions primarily by replenishing intracellular glutathione and restoring redox balance.[9] In cases of drug-induced liver injury (DILI), particularly those associated with first-line anti-tuberculosis therapy (ATT), oxidative stress is a major contributor to hepatocyte damage. In India, where tuberculosis remains highly prevalent, ATT-induced liver injury is a major concern. Clinical trials have shown that adjunctive use of NAC during ATT can reduce the incidence and severity of liver enzyme elevations, suggesting a hepatoprotective role.[10] Yet, despite promising findings, a standardized preventive approach for ATT-related hepatotoxicity is still lacking, and further research is needed to validate NAC’s efficacy in routine clinical settings.[11]
Alcohol-induced liver disease presents yet another significant etiology of hepatic injury. ALD is a global public health burden, contributing to over 200 disease conditions and accounting for approximately 6% of global deaths. Chronic alcohol consumption results in a spectrum of liver pathology, from fatty liver to hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma.[12] Ethanol metabolism generates toxic intermediates such as acetaldehyde and promotes oxidative stress via cytochrome P450 enzyme pathways, leading to hepatocellular inflammation and fibrosis.[13] Additionally, alcohol impairs gut barrier function, allowing bacterial endotoxins to reach the liver and trigger further injury through inflammatory cascades.[14]
In addressing ALD, botanical agents with antioxidant and anti-inflammatory properties offer promising alternatives to synthetic drugs, which often carry long-term safety concerns. Curcumin, the active component of turmeric, has long been recognized for its broad pharmacological activity, including hepatoprotective effects. However, its poor bioavailability limits its clinical utility.[15] Novel formulations like the CGM have demonstrated enhanced absorption and therapeutic potential in reducing hepatic enzyme elevations, oxidative stress, and inflammatory markers in patients with alcohol-induced liver dysfunction. These findings position CGM as a viable nutraceutical intervention in managing ALD.[16]
In parallel, acute liver failure (ALF) is often triggered by paracetamol overdose or idiosyncratic drug reactions and remains a medical emergency with limited treatment options beyond liver transplantation.[17] The pathogenesis of ALF involves a cascade of hepatocellular necrosis, immune cell infiltration, and systemic inflammation. While NAC remains the standard of care for paracetamol-induced ALF when administered early, its efficacy wanes with delayed treatment. For non-paracetamol ALF, effective therapies are virtually nonexistent.[18]
In this context, cell-based immunotherapy has emerged as a novel approach. Macrophage therapy, particularly using alternatively activated macrophages (AAMs), offers a mechanistically distinct strategy by promoting tissue repair, phagocytosis of necrotic cells, and anti-inflammatory signaling.[19] Early-phase human trials in chronic liver disease have demonstrated the safety and feasibility of autologous macrophage infusions. The MAIL macrophage therapy for acute injury to the Liver (MAIL) study represents a significant step toward evaluating allogeneic AAMs for ALF, particularly in paracetamol-related cases, with the aim of preventing progression of liver failure and reducing the need for transplantation.[20]
Collectively, these studies underscore the multifaceted nature of liver toxicity, encompassing metabolic, infectious, pharmacological, and lifestyle-related insults.[21] The evolving understanding of pathophysiological mechanisms, especially those involving oxidative stress, inflammation, and the gut-liver axis, has paved the way for innovative therapies, ranging from microbiota-targeting interventions to antioxidant supplementation and cellular immunotherapy.[22] As liver toxicity continues to pose a global health threat, especially in resource-limited settings, such multidisciplinary strategies hold promise for both prevention and treatment across diverse liver disease spectrums.[23,24]
This study aims to explore emerging therapeutic strategies for liver toxicity of multifactorial origin, focusing on interventions that target oxidative stress, inflammation, and gut-liver axis dysregulation. It evaluates the potential of probiotics, NAC, CGM complex, and macrophage-based cell therapy across various liver disease etiologies to identify scalable, effective treatments, particularly for high-risk and resource-limited populations.
Methodology
A systematic literature search was conducted using PubMed and Google Scholar databases to identify relevant studies addressing post-surgical pain following breast cancer treatment. The search strategy incorporated the terms: “liver toxicity” AND “hepatoprotective”, and filters were applied to include clinical trials, randomized controlled trials, observational studies, and human Studies. The search included publications from January 1, 2015, to June 30, 2025, and was limited to studies published in English.
Inclusion criteria:
- Clinical trials, observational studies, and randomized controlled trials
- Studies involving human subjects
- Articles published in English
- Studies including male and female participants
- Studies were published between January 1, 2015, and June 30, 2025
Exclusion criteria:
- Books, commentaries, editorials, letters, documents, and book chapters
- Case reports, case series, and literature reviews
- Articles published in languages other than English
- Animal studies and in vitro (laboratory) studies
- Articles published before January 1, 2015, or after June 30, 2025
- Studies lacking a reported results section
The initial search yielded a total of 11020 articles. After applying the inclusion and exclusion criteria, 1666 references were downloaded from PubMed and Google Scholar. Prior to screening, 1450 records were removed due to duplication, technical errors, or irrelevance. In the screening phase, the remaining 216 records were assessed based on titles and abstracts, resulting in the exclusion of 148 studies. Of the 68 reports selected for retrieval, 54 could not be accessed due to the unavailability of full text or restricted access.
The remaining 14 full-text articles were evaluated for eligibility. Of these, 10 studies were excluded due to a scale for the assessment of narrative review articles (SANRA) score of <10, indicating insufficient quality. Ultimately, four studies met all inclusion criteria and were included in the final review.
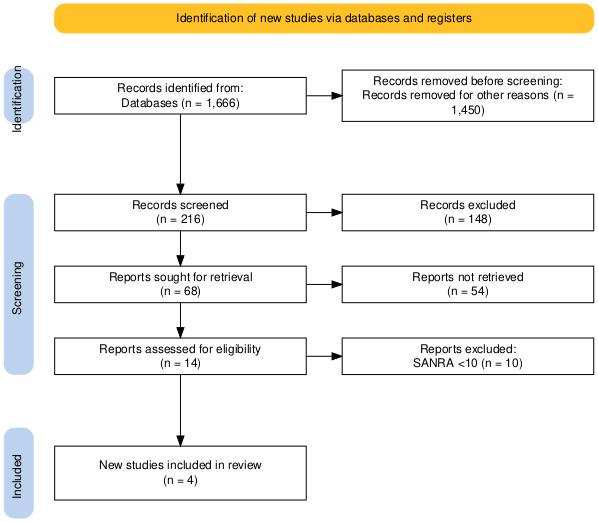
Figure 1: PRISMA flow diagram
Results
Effect of probiotics on liver toxicity during antidepressant use
Gawlik-Kotelnicka O et al. demonstrated their study which included two groups: a probiotic (PRO) group and a placebo (PLC) group. Due to a moderately high dropout rate, there are eight participants in the PRO group and nine in the PLC group, and the analysis was conducted per protocol rather than by intention-to-treat.
Baseline characteristics across both groups were comparable. Most participants were on psychotropic medications, predominantly selective serotonin reuptake inhibitors (SSRIs) such as sertraline (n = 20) and escitalopram (n = 12). Other medications included duloxetine (n = 9), trazodone (n = 11), pregabalin (n = 9), and lamotrigine (n = 6). Polypharmacy was common, with 29 individuals regularly using at least two psychotropic drugs. Additionally, four participants (two in each group) were on metformin, known to influence gut microbiota.
Probiotic supplementation did not result in significant changes in non-invasive biomarkers of liver steatosis or fibrosis in the general cohort. The framingham steatosis index (FSI) was the only marker to show a significant overall reduction from baseline (Median (interquartile range (IQR)): −2.64 (−3.50 to −1.59) to −2.82 (−3.61 to −1.89); p < 0.001), although the effect was similar between the PRO and PLC groups (p = 0.974).
Subgroup analyses provided additional insights. Among participants diagnosed with a depressive episode (DE), probiotic intervention was associated with greater improvements in liver steatosis markers compared to those with mixed depression and anxiety disorder (MDAD). Alanine Aminotransferase/Aspartate Aminotransferase (ALT/AST) ratios declined by −4.5% (IQR): −27.9 to 7.1) in the DE group versus an increase of 10.7% (IQR: 0.3 to 19.4) in the MDAD group (p = 0.011), while Hepatic Steatosis Index (HSI) values showed a decrease of −0.3% (IQR: −6.5 to 1.7) compared to a 3% increase (IQR: −0.9 to 4.9) (p = 0.022).
Notably, DE participants were more likely to fulfill the glycemic criterion of metabolic syndrome (27.3% vs. 7.4%, p = 0.061) and were more frequently prescribed SSRIs (82.3% vs. 50%, p = 0.049), though total antidepressant use did not significantly differ (77.3% vs. 59.2%, p = 0.181).
Psychotropic medication use independently influences liver outcomes. In the PRO group, those on such medications showed greater reductions in ALT (−3.9% (IQR: −24.8 to 8.6)) compared to those without (9% (IQR: 1.8 to 41.2); p = 0.056). ALT (p = 0.032) and ALT/AST ratio (p = 0.051) also showed global significance when stratified by medication use. Conversely, fibrosis-4 index (FIB-4) scores improved more in the MDAD subgroup than in DE (4.3% vs. 10.7%; p = 0.028), independent of antidepressant therapy.
While metabolic syndrome and depressive severity did not significantly influence liver outcomes, the presence of chronic low-grade inflammation (CLGI) appeared to impact AST to platelet ratio index (APRI) (global p = 0.028), though small subgroup sizes limited statistical power.
Finally, a significant correlation between improvement in depressive symptoms and reductions in liver steatosis markers was observed in the PRO group but not the PLC group. Baseline HSI and FSI were also significantly correlated with CRP levels (r = 0.39, p = 0.006; r = 0.47, p < 0.001, respectively), suggesting an inflammatory link. No associations were found with changes in quality of life.[25]
Effect of NAC on liver toxicity and oxidative stress
According to the study conducted by Sukumaran et al., baseline characteristics were comparable between the two groups, ensuring homogeneity at the start of the study. In the NAC group, a statistically significant reduction was observed in liver function parameters at 4 weeks, including alanine aminotransferase (ALT, p < 0.01), alkaline phosphatase (ALP, p < 0.01), and total bilirubin (p < 0.001) when compared to baseline values.
Other biomarkers, namely aspartate aminotransferase (AST), malondialdehyde (MDA), and nitric oxide (NO), which also showed reductions from baseline levels by 19%, 21.6%, and 5.5%, respectively, while glutathione (GSH) increased by 2.6%. However, these changes did not reach statistical significance. Notably, these trends in liver function and oxidative stress markers were maintained through the 8-week period, indicating a sustained effect of NAC over time.
Quality of life (QOL) scores improved significantly from baseline in both groups (p < 0.05), suggesting a general positive clinical response irrespective of treatment type. However, between-group analysis revealed a significantly greater reduction in ALT (p < 0.05) and AST (p < 0.05) in the NAC group at 4 weeks compared to the placebo group. Improvements were also noted in bilirubin, MDA, NO, and GSH levels in the NAC group versus placebo at the same time point, though these between-group differences were not statistically significant.
By 8 weeks, the NAC group continued to show favorable trends in liver function tests (LFTs) and oxidative stress markers compared to placebo, further supporting the potential therapeutic benefit of NAC. These effects appeared consistently and stably over the treatment duration.
Adverse drug reactions (ADRs) such as itching and rashes were the most reported in both groups, with a similar incidence rate, indicating no increased risk associated with NAC use. Compliance with the study protocol was high in both treatment arms, contributing to the reliability of the results.
In summary, NAC demonstrated statistically significant improvements in key liver function parameters and sustained favorable trends in oxidative stress markers. While some changes were not statistically significant, the consistent pattern across multiple indicators, along with good tolerability and adherence, supports the potential benefit of NAC in improving hepatic and oxidative profiles.[26]
Effect of CGM on liver toxicity and oxidative stress
T Krishnareddy N et al. demonstrated in a study involving 48 participants who were randomly assigned into two groups: a placebo group and a group receiving CGM, with 24 individuals in each. The mean age in the placebo group was 38.6 ± 3.92 years, while in the CGM group it was 40 ± 3.1 years. At baseline, both groups were comparable in terms of anthropometric measurements, body composition, and blood pressure. Over the course of the study, three participants discontinued from the placebo group and one from the CGM group due to non-compliance, primarily linked to difficulty abstaining from alcohol, not due to adverse reactions. Initial biochemical profiles, including liver enzymes such as AST, ALT, and ALP, were similar between the two groups (p > 0.05), and all other hematological and biochemical indicators remained within healthy reference ranges.
Identical-appearing CGM and placebo capsules (250 mg ×1) were used in the intervention, and both were validated for content and quality through high-performance liquid chromatography (HPLC) and high-performance thin-layer chromatography (HPTLC) methods. The CGM formulation contained 39.1% total curcuminoids with curcumin, demethoxycurcumin, and bis-demethoxycurcumin present in a ratio of 77.4:14.7:3.2. The placebo was composed of microcrystalline cellulose (MCC) with food-grade coloring to match the CGM’s appearance.
Liver enzyme levels (ALT, aspartate aminotransferase (AST), ALP, and gamma-glutamyl transferase (GGT)) were measured at baseline, Day 28, and Day 56. At the start, all participants had significantly elevated liver enzyme levels (p < 0.01), but no difference between groups. Over time, the placebo group showed a consistent increase in liver enzymes, indicative of progressive hepatic dysfunction. Conversely, the CGM group experienced marked reductions. ALT increased by 4.02% and 7.8% in the placebo group on Days 28 and 56, respectively, but decreased by 16.5% and 36% in the CGM group (p < 0.001). GGT rose by 6.75% to 11.8% in the placebo group yet declined by 16% and 29% in the CGM group (p < 0.001). AST and ALP followed similar trajectories, increasing in the placebo group while significantly decreasing in the CGM group (p < 0.01). Overall, CGM led to an average 32% reduction in liver enzyme levels, compared to a 13.4% increase in placebo. Between-group comparisons showed a 38.6% greater reduction in liver enzymes for CGM.
Oxidative stress markers were also significantly influenced by CGM. Reduced Glutathione (GSH) levels declined by 21.2% in the placebo group but increased by 19.6% in the CGM group (p < 0.001). Superoxide dismutase (SOD) activity rose by 23.4% in CGM and declined by 11.3% in placebo (p < 0.001). Glutathione peroxidase (GPx) decreased by 17% in the placebo group but improved by 24.8% with CGM supplementation. Lipid peroxidation, assessed via thiobarbituric acid reactive substances (TBARS), increased by 12% in the placebo group and decreased by 18.9% in the CGM group (p < 0.001), indicating reduced oxidative damage.
Inflammatory markers, interleukin-6 (IL-6) and C-reactive protein (CRP), also differed markedly between groups over the 56-day period. Although initial values were not statistically different (p > 0.05), CRP increased by 34% and IL-6 by 16.1% in the placebo group, signaling escalating inflammation. In contrast, the CGM group showed significant reductions in both markers, with CRP dropping by 27.3% and IL-6 by 15.1% from baseline (p < 0.01). These findings were statistically significant for both within-group and between-group comparisons, supporting the anti-inflammatory potential of CGM supplementation.[27]
Effect of infusion-based intervention to treat paracetamol-induced liver toxicity
Humphries C et al. demonstrated that the primary outcome of the study was the occurrence of dose-limiting toxicity (DLT) within 30 days following infusion. DLT was defined as any clinically significant adverse event or abnormal laboratory value that was not attributable to disease progression, intercurrent illness, or concomitant medications. Events were classified as DLT if they met the National Cancer Institute common terminology criteria for adverse events (CTCAE) Grade 3, 4, or 5, or if they were determined by the independent data monitoring committee (DMC) to be sufficiently serious to preclude dose escalation.
Secondary outcomes were evaluated within 30 days post-dosing and included assessments of safety, adverse events, pharmacodynamic activity, and immunogenicity. Safety was evaluated through clinical observations, physical examinations, electrocardiograms (ECGs), and routine safety laboratory tests conducted on days 0, 1, 2, 7, and 30. Particular attention was paid to adverse events of special interest, specifically transfusion reactions, macrophage activation syndrome, and acute respiratory compromise, in addition to all serious adverse events (SAEs) occurring within the 30-day period.
Pharmacodynamic activity was assessed on the same schedule (days 0, 1, 2, 7, and 30), with additional exploratory analyses performed if stored serum samples from earlier time points were available. Biomarkers of inflammation and liver injury were analyzed to evaluate treatment response. Proinflammatory cytokines—including interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), IL-12, and IL-8—were measured in pg/mL, while IL-10 served as an anti-inflammatory marker. All cytokines were assessed as changes from baseline.
Markers of liver injury included both conventional and novel parameters. Conventional markers encompassed alanine aminotransferase (ALT, U/L), international normalised ratio (INR), lactate (mmol/L), and creatinine (μmol/L), all commonly associated with paracetamol-induced liver injury. In addition, novel biomarkers such as high mobility group box one protein (HMGB1, ng/mL), glutamate dehydrogenase (GDH, U/L), cytokeratin-18 (CK-18, U/L), and microRNA-122 (miR-122, copies/mL) were assessed to provide further insight into liver cell injury. All liver injury parameters were evaluated as changes from baseline values.
Immunogenicity was examined on day 30 by testing for the development of anti-human leukocyte antigen (HLA) antibodies, which could indicate an immune response against the therapeutic intervention.[28]
| Study | Sample size | Design | Population | Key findings | Limitations |
| Gawlik-Kotelnicka O,et al.[25] | 17 (8 Probiotic, 9 Placebo) | Randomized, double-blind, per-protocol analysis | Patients with depressive and/or anxiety disorders on psychotropic medications | – No significant difference in general liver markers between groups – Subgroup (Depressive Episode) showed improved ALT/AST ratio and HSI with probiotics – Improvements in liver steatosis correlated with better depressive symptoms and CRP reduction | – Small sample size – High dropout rate – Lack of intention-to-treat analysis – Limited statistical power for subgroup analyses |
| Sukumaran D,et al.[26] | 60 (30 NAC, 30 Placebo) | Prospective, randomized, controlled trial | Patients receiving first-line anti-tuberculosis therapy (ATT) | – Significant improvements in ALT, ALP, and bilirubin at 4 weeks in the NAC group – Favorable trends in oxidative stress markers (↑GSH, ↓MDA, NO) – Good tolerability and compliance | – Changes in some oxidative markers not statistically significant – Limited duration (8 weeks) – Modest sample size |
| T Krishnareddy N et al.[27] | 48 (24 CGM, 24 Placebo) | Randomized, double-blind, placebo-controlled trial | Patients with alcohol-induced liver dysfunction | – Significant reductions in ALT, AST, ALP, GGT (up to 36%) – Improved oxidative stress markers (↑GSH, SOD; ↓TBARS) – Decreased inflammatory markers (↓CRP, IL-6) vs. placebo | – Short duration (56 days) – Modest sample size – Dropouts due to alcohol non-compliance |
| Humphries C, et al.[28] | Not clearly specified (Early phase trial) | Phase I dose-escalation study | Patients with acute liver failure (ALF), especially due to paracetamol | – No dose-limiting toxicity (DLT) identified – Favorable safety profile – Reductions in liver injury markers (ALT, INR, lactate, novel biomarkers like HMGB1, miR-122) – Anti-inflammatory cytokine (IL-10) increased | – Early-phase with safety focus – Small/unspecified sample size – No control group – Limited long-term outcome data |
Table 1: Comparative table of studies on emerging therapies for hepatotoxicity
Discussion
Liver toxicity represents a global health burden arising from diverse etiologies, including metabolic syndromes, drug exposure, alcohol use, and immune dysregulation. Recent research has illuminated both the common pathways and unique mechanisms by which these insults result in hepatocellular injury. This review comparatively explores emerging therapeutic strategies such as probiotics, N-acetylcysteine (NAC), curcumin-galactomannoside complex (CGM), and macrophage-based cell therapy, based on clinical data from Gawlik-Kotelnicka et al., Sukumaran et al., Krishnareddy et al., and Humphries et al.
Gawlik-Kotelnicka et al. investigated the effects of probiotics in individuals with depressive disorders, many of whom were on psychotropic medications known to influence metabolic and hepatic health. While overall changes in liver steatosis and fibrosis markers were not statistically significant, probiotics showed promise in specific subgroups, particularly those with major depressive episodes (DE). ALT/AST ratios and hepatic steatosis indices improved significantly in the DE subgroup, suggesting a potential interaction between mood pathology, systemic inflammation, and liver function. The study supports the hypothesis that gut microbiota modulation can indirectly affect hepatic health, especially in the context of inflammation-related mood disorders. Interestingly, liver biomarkers correlated with CRP levels, reinforcing the inflammatory link. However, the small sample size and high dropout rate limit generalizability.[25]
In contrast, Sukumaran et al. provided robust evidence for NAC’s hepatoprotective effect in patients on first-line anti-tuberculosis therapy (ATT), a well-known cause of drug-induced liver injury (DILI). NAC significantly reduced ALT, ALP, and bilirubin levels after four weeks and maintained these trends over eight weeks. Although reductions in oxidative stress markers like MDA and nitric oxide were not statistically significant, their consistent decline supports NAC’s antioxidative potential. Importantly, improvements in liver function were observed alongside enhanced quality of life, without a significant increase in adverse reactions. These findings are in line with previous literature suggesting that NAC replenishes intracellular glutathione, neutralizes free radicals, and provides a protective buffer against hepatic oxidative stress. Unlike probiotics, NAC’s benefit was clearer and more uniform across the cohort, potentially due to its direct antioxidative mechanism.[26]
Krishnareddy et al. demonstrated that the CGM formulation had both hepatoprotective and anti-inflammatory effects in individuals with alcohol-induced liver dysfunction. Over 56 days, CGM significantly reduced liver enzyme levels (ALT, AST, ALP, and GGT) compared to placebo. Oxidative stress parameters (GSH, SOD, GPx) improved, while TBARS, a marker of lipid peroxidation, decreased significantly. CGM also reduced pro-inflammatory markers (CRP and IL-6), highlighting its systemic anti-inflammatory potential. These effects were consistent and more pronounced than those seen with NAC or probiotics, suggesting CGM’s dual antioxidative and anti-inflammatory action may offer superior protection in ALD. However, long-term studies are needed to confirm its safety and efficacy, especially given the metabolic complexities in alcohol-dependent populations.[27]
Humphries et al. introduced a novel, cell-based approach using allogeneic alternatively activated macrophages (AAMs) for treating paracetamol-induced acute liver injury (ALI). This phase 1 trial primarily focused on safety and tolerability. While detailed efficacy outcomes are pending, the infusion showed a favorable safety profile with no dose-limiting toxicity observed. The study also monitored changes in liver injury markers and inflammatory cytokines (e.g., IL-6, tumor necrosis factor(TNF)-α), offering mechanistic insight into how macrophages could promote hepatic repair through anti-inflammatory and regenerative signaling. This therapeutic approach is fundamentally different from NAC or nutraceuticals like CGM. Rather than preventing injury via antioxidant mechanisms, AAMs actively contribute to the resolution of inflammation and tissue remodeling, potentially filling the treatment gap for patients who present late in the ALI continuum when NAC is no longer effective.[28]
When compared across interventions, NAC and CGM offer the most immediate clinical translation potential, with strong evidence of biochemical and clinical improvement and favorable safety profiles. NAC is particularly well-suited for DILI due to oxidative stress, while CGM may be more effective in lifestyle-related hepatic injuries like ALD due to its broader anti-inflammatory reach. Probiotics offer a more targeted effect in specific populations, particularly those with mood disorders and underlying inflammation, though results remain heterogeneous. Meanwhile, macrophage therapy, though still investigational, represents a cutting-edge approach to reversing established liver damage, and its success could pave the way for immunotherapeutic strategies in hepatology.[29]
Liver toxicity requires a multifaceted therapeutic framework tailored to the underlying cause, be it metabolic, pharmacological, or inflammatory. While antioxidants and nutraceuticals like NAC and CGM show clear benefits, personalized interventions such as microbiome modulation and cell therapy may further refine treatment algorithms. Future research must focus on larger, controlled trials, mechanistic validation, and cost-effective scalability to integrate these findings into clinical practice.[30]
Cost, availability, and scalability of emerging therapies
While the therapeutic efficacy of emerging hepatoprotective interventions such as NAC, probiotics, CGM, and macrophage-based immunotherapy is increasingly supported by clinical data, their real-world implementation is influenced by factors like cost, market availability, and scalability, especially in resource-limited settings.
N-Acetylcysteine:
- Cost: NAC is one of the most cost-effective therapies in this review. Generic formulations are widely available, and a full course for liver protection costs significantly less than most proprietary formulations or biological therapies.
- Availability: It is widely available worldwide, especially in both high-income and low- to middle-income countries (LMICs), due to its long-standing use in acetaminophen overdose and mucolytic therapy.
- Scalability: The production and distribution infrastructure is already established, making it highly scalable for mass use in hepatotoxicity prevention, especially in TB-endemic regions where ATT-induced liver injury is prevalent.
Probiotics:
- Cost: Probiotics range from moderately priced to expensive, depending on the strain diversity and formulation (e.g., enteric-coated capsules, spore-based).
- Availability: Over-the-counter availability is increasing, but standardization and regulation vary greatly across countries. In LMICs, high-quality, clinically validated probiotic strains may not be easily accessible.
- Scalability: While manufacturing scalability is feasible, efficacy is strain- and population-dependent, limiting broad policy-level adoption without localized clinical validation. Further, cold-chain logistics are often required, complicating rural distribution.
CGM:
- Cost: CGM is a proprietary formulation and thus more expensive than plain curcumin or NAC. Its enhanced bioavailability justifies the cost from a pharmacokinetic standpoint, but this may limit its widespread adoption in low-resource settings.
- Availability: It is not yet widely available globally and is currently limited to specific markets, primarily as a nutraceutical. Regulatory approval varies, and in some countries, CGM is not yet registered as a therapeutic product.
- Scalability: Manufacturing CGM requires advanced formulation technology (e.g., HPTLC and HPLC-validated processes), which can limit large-scale production. While promising, policy-level integration would require cost-reduction strategies, local production, or subsidized programs in LMICs.
Macrophage-Based Therapy:
- Cost: This therapy is significantly cost-prohibitive at present. Cell isolation, ex vivo manipulation, quality control, and sterile infusion facilities incur high upfront and maintenance costs, putting them out of reach for routine clinical use.
- Availability: As an investigational therapy, it is currently available only within clinical trial settings or specialized centers in high-income countries. No commercial formulations exist yet.
- Scalability: Scalability remains the most substantial challenge. Cell-based therapies require personalized or semi-personalized approaches, good manufacturing practices (GMP)-compliant facilities, trained personnel, and sophisticated cold-chain systems. In the short term, scaling to national or international levels is unfeasible without massive infrastructural and economic investment.
Conclusion
Liver toxicity remains a significant global health concern, with diverse etiologies including metabolic dysfunction, alcohol use, drug-induced damage, and immune-mediated mechanisms. Recent research highlights the potential of targeted interventions such as probiotics, NAC, CGM, and macrophage-based therapies in mitigating liver injury through anti-inflammatory, antioxidant, and microbiota-modulating effects. Probiotics offer dual benefits in patients with depression and liver dysfunction by acting on the gut-liver-brain axis. NAC demonstrates protective effects against oxidative stress, especially in drug-induced liver injury, while CGM significantly improves liver enzyme profiles and reduces oxidative and inflammatory markers in alcohol-related liver disease. Macrophage therapy presents a novel regenerative strategy, particularly in acute liver failure. Despite promising findings, further large-scale, multicenter trials are needed to establish standardized, effective, and safe treatment protocols. Integrating these interventions into personalized, mechanism-based strategies could enhance therapeutic outcomes and reduce the global burden of hepatotoxicity.
References
- Thakur S, Kumar V, Das R, et al. Biomarkers of hepatic toxicity: an overview. Curr Ther Res Clin Exp. 2024;100:100737. doi:10.1016/j.curtheres.2024.100737 PubMed | Crossref | Google Scholar
- Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. 2024;29(1):101133. doi:10.1016/j.aohep.2023.101133
PubMed | Crossref | Google Scholar - Rinella ME, Sookoian S. From NAFLD to MASLD: updated naming and diagnosis criteria for fatty liver disease. J Lipid Res. 2024;65(1):100485. doi:10.1016/j.jlr.2023.100485
PubMed | Crossref | Google Scholar - Weinstein AA, De Avila L, Kannan S, et al. Interrelationship between physical activity a nd depression in nonalcoholic fatty liver disease. World J Hepatol. 2022;14(3):612-622. doi:10.4254/wjh.v14.i3.612
PubMed | Crossref | Google Scholar - Hrncir T. Gut microbiota dysbiosis: triggers, consequences, diagnostic and therapeutic options. Microorganisms. 2022;10(3):578. doi:10.3390/microorganisms10030578
PubMed | Crossref | Google Scholar - Liu Q, Wang S, Fu J, et al. Liver regeneration after injury: Mechanisms, cellular interactions and therapeutic innovations. Clin Transl Med. 2024;14(8):e1812. doi:10.1002/ctm2.1812
PubMed | Crossref | Google Scholar - Chandrasekaran P, Weiskirchen S, Weiskirchen R. Effects of Probiotics on Gut Microbiota: An Overview. Int J Mol Sci. 2024;25(11):6022. doi:10.3390/ijms25116022
PubMed | Crossref | Google Scholar - Ng QX, Peters C, Ho CYX et al. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord. 2018;228:13-19. doi:10.1016/j.jad.2017.11.063
PubMed | Crossref | Google Scholar - Jaeschke H, Akakpo JY, Umbaugh DS, Ramachandran A. Novel therapeutic approaches against acetaminophen-induced liver injury and acute liver failure. Toxicol Sci. 2020;174(2):159-167. doi:10.1093/toxsci/kfaa002
PubMed | Crossref | Google Scholar - Kumar R, Kumar A, Patel R, et al. Incidence and risk factors of antituberculosis drug-induced liver injury in India: A systematic review and meta-analysis. Indian J Gastroenterol. 2025;44(1):35-46. doi:10.1007/s12664-024-01643-w
PubMed | Crossref | Google Scholar - AbdulRaheem Y. Unveiling the Significance and Challenges of Integrating Prevention Levels in Healthcare Practice. J Prim Care Community Health. 2023;14:21501319231186500. doi:10.1177/21501319231186500
PubMed | Crossref | Google Scholar - Mirowski K, Balicka-Ślusarczyk B, Hydzik P et al. Alcohol-associated liver disease – a current overview. Folia Med Cracov. 2024;64(2):93-104. doi:10.24425/fmc.2024.150156
PubMed | Crossref | Google Scholar - Lang AL, Beier JI. Interaction of volatile organic compounds and underlying liver disease: a new paradigm for risk. Biol Chem. 2018;399(11):1237-1248. doi:10.1515/hsz-2017-0324
PubMed | Crossref | Google Scholar - Contreras-Zentella ML, Villalobos-García D, Hernández-Muñoz R. Ethanol metabolism in the liver, the induction of oxidant stress, and the antioxidant defense system. Antioxidants (Basel). 2022;11(7):1258. doi:10.3390/antiox11071258
PubMed | Crossref | Google Scholar - Sharifi-Rad J, Rayess YE, Rizk AA, et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol. 2020;11:01021. doi:10.3389/fphar.2020.01021
PubMed | Crossref | Google Scholar - Pancholi V, Smina TP, Kunnumakkara AB et al. Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study. Toxicol Rep. 2021;8:1255-1264. doi:10.1016/j.toxrep.2021.06.008
PubMed | Crossref | Google Scholar - Arshad MA, Bangash MN. Acute liver failure following paracetamol overdose. J Intensive Care Soc. 2022;23(2):244-251. doi:10.1177/17511437211007777
PubMed | Crossref | Google Scholar - Nabi T, Nabi S, Rafiq N et al. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: A prospective study. Saudi J Gastroenterol. 2017;23(3):169-175. doi:10.4103/1319-3767.207711
PubMed | Crossref | Google Scholar - Humphries C, Addison ML, Dear JW et al. The emerging role of alternatively activated macrophages to treat acute liver injury. Arch Toxicol. 2025;99(1):103-114. doi:10.1007/s00204-024-03892-2
PubMed | Crossref | Google Scholar - Enoghase R. J, Osarinmwian I. B, Osegbe E. D, Abu J, Abukadiri M, Ajufoh C. Z. Hepatoprotective Effects of Lawsonia inermis Aqueous Extract Against Lead Acetate-Induced Liver Injury in Wistar Rats. medtigo J Med. 2024;2(4):e30622418. doi:10.63096/medtigo30622418
Crossref | Google Scholar - Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184-6223. doi:10.3390/ijms15046184
PubMed | Crossref | Google Scholar - Wang L, Cheng CK, Yi M, Lui KO, Huang Y. Targeting endothelial dysfunction and inflammation. J Mol Cell Cardiol. 2022;168:58-67. doi:10.1016/j.yjmcc.2022.04.011
PubMed | Crossref | Google Scholar - Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79(2):516-537. doi:10.1016/j.jhep.2023.03.017
PubMed | Crossref | Google Scholar - Idilman R, Aydogan M, Oruncu MB, et al. Natural History of Cirrhosis: Changing Trends in Etiology Over the Years. Dig Dis. 2021;39(4):358-365. doi:10.1159/000512746
PubMed | Crossref | Google Scholar - Gawlik-Kotelnicka O, Burzyński J, Rogalski J et al. Probiotics may be useful for drug-induced liver dysfunction in patients with depression – A secondary analysis of a randomized clinical trial. Clin Nutr ESPEN. 2024;63:604-614. doi:10.1016/j.clnesp.2024.07.1024
PubMed | Crossref | Google Scholar - Sukumaran D, Usharani P, Paramjyothi GK et al. A study to evaluate the hepatoprotective effect of N- acetylcysteine on anti tuberculosis drug induced hepatotoxicity and quality of life. Indian J Tuberc. 2023;70(3):303-310. doi:10.1016/j.ijtb.2022.05.012
PubMed | Crossref | Google Scholar - Krishnareddy T, Thomas JV, Nair SS, et al. A novel curcumin-galactomannoside complex delivery system improves hepatic function markers in chronic alcoholics: a double-blinded, randomized, placebo-controlled study. Biomed Res Int. 2018;2018:9159281. doi:10.1155/2018/9159281
PubMed | Crossref | Google Scholar - Asgarshirazi M, Shariat M, Sheikh M. Comparison of efficacy of folic acid and silymarin in the management of antiepileptic drug induced liver injury: a randomized clinical trial. Hepatobiliary Pancreat Dis Int. 2017;16(3):296-302. doi:10.1016/s1499-3872(16)60142-x
PubMed | Crossref | Google Scholar - Paik JM, Golabi P, Younossi Y et al. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology. 2020;72(5):1605-1616. doi:10.1002/hep.31173
PubMed | Crossref | Google Scholar - Xie C, Singal AK. Global burden of cirrhosis and liver cancer due to alcohol: the past, present, and the future. Hepatol Int. 2023;17(4):830-832. doi:10.1007/s12072-023-10534-9
PubMed | Crossref | Google Scholar
Acknowledgments
Not applicable
Funding
Not applicable
Author Information
Corresponding Author:
Samatha Ampeti
Department of Pharmacology
Kakatiya University, University College of Pharmaceutical Sciences, Warangal, TS, India
Email: ampetisamatha9@gmail.com
Co-Authors:
Sonam Shashikala BV, Mansi Srivastava, Shubham Ravindra Sali, Nirali Patel, Raziya Begum Sheikh
Independent Researcher
Department of Content, medtigo India Pvt Ltd, Pune, India
Ethical Approval
Not applicable
Conflict of Interest Statement
None
Guarantor
None
DOI
Cite this Article
Sonam SBV, Samatha A, Mansi S, Shubham RS, Nirali P, Raziya BS. A Systematic Review on Emerging Therapies for Liver Toxicity. medtigo J Pharmacol. 2025;2(3):e3061237. doi:10.63096/medtigo3061237 Crossref









