Author Affiliations
Abstract
This Quality Improvement Project (QIP) aimed to enhance the management of anticoagulation therapy in atrial fibrillation (AF) patients by improving adherence to the CHA2DS2-VA and HAS-BLED risk assessment scores. Recognizing that AF significantly elevates stroke risk, our initiative began with a clinical audit involving 50 patients presenting with new or paroxysmal AF. Initial findings revealed that only 27% and 5% of patients had their CHA2DS2-VA and HAS-BLED scores calculated, respectively, with 42% receiving anticoagulants, of which only 20% were correctly dosed. Following this, a comprehensive action plan was implemented, emphasizing education through posters, emails, and presentations, targeting both junior and senior doctors. A follow-up audit conducted three months later demonstrated substantial improvements100% compliance in score calculations and anticoagulant prescriptions, alongside 100% correct dosing. These results underscore the project’s success in aligning clinical practices with international guidelines, significantly enhancing patient safety and treatment outcomes. The project’s success highlights the effectiveness of structured educational initiatives in enhancing clinical practice, aligning anticoagulation management with National Institute for Health and Clinical Excellence (NICE) and European Society of Cardiology (ESC) guidelines, and ultimately improving patient safety and outcomes. Ongoing education and monitoring are vital to maintain these gains and further improve anticoagulation management for AF patients, thereby reducing the risk of adverse events and optimizing healthcare delivery.
Keywords
Atrial fibrillation, Anticoagulation, Stroke, Quality Improvement Project, Audit.
Introduction
A QIP focusing on the clinical audit and re-audit of anticoagulation use in AF patients aims to ensure that clinical practices align with international guidelines and improve patient outcomes. AF is a major risk factor for stroke, and patients with AF who experience strokes often face greater long-term disability compared to other stroke patients.[5] Anticoagulants are crucial in reducing both stroke risk and mortality in AF patients, with greater benefits for those at higher risk. The CHA2DS2-VA score is used to predict stroke risk, while the HAS-BLED score assesses bleeding risk associated with anticoagulant therapy. The ESC and the NICE recommend calculating these scores for all non-valvular AF patients to guide anticoagulation decisions. Despite these guidelines, our initial audit revealed underutilization of CHA2DS2-VA and HAS-BLED scores, which may reflect inadequate prescription of oral anticoagulants. To address this, we designed a QIP consisting of two cycles over six months, initially focusing on raising awareness among junior and senior doctors about risk assessment and the importance of appropriate anticoagulation prescription through educational interventions such as posters, emails, and presentations. The second cycle demonstrated improvements in the rates of CHA2DS2-VA and HAS-BLED score assessments, highlighting the project’s impact on enhancing clinical practice. The primary objective of this QIP is to enhance the assessment of stroke and bleeding risks in patients with AF by increasing the utilization of the CHA2DS2-VA and HAS-BLED scores. These scores are essential tools for predicting stroke risk and bleeding risk, respectively, and their proper use is crucial for making informed decisions about anticoagulant therapy. The secondary objective is to improve the prescription practices of oral anticoagulants by ensuring that these medications are prescribed based on comprehensive risk assessments. By achieving these objectives, the project aims to align clinical practices with international guidelines, ultimately enhancing patient safety and treatment outcomes in AF management.
Methodology
An initial audit was conducted to evaluate the use of CHA2DS2-VA and HAS-BLED scores for patients with non-valvular AF and the appropriateness of anticoagulation prescriptions in patients with AF, mitral stenosis in rheumatic heart diseases, and patients with metallic prosthetic valves. This audit, covering 50 patients presenting to acute care units with either new or paroxysmal AF from March 2024 to August 2024, utilized retrospective data from medical health records. The audit tool focused on three main areas: the calculation of CHA2DS2-VA scores, the calculation of HAS-BLED scores, and the appropriateness of anticoagulant prescriptions, including dosage accuracy. The minimum accepted compliance rates were set at 90% for both CHA2DS2-VA and HAS-BLED scores and 100% for correct anticoagulant prescriptions.[2] Results revealed that CHA2DS2-VA and HAS-BLED scores were calculated in only 40% and 30% of patients, respectively, with anticoagulants prescribed to 42% of patients, only 20% of whom received the correct dosage, while 38% did not receive any anticoagulants. The audit highlighted several issues, including high workloads, under-documentation of risk scores, and a lack of awareness regarding international guidelines and local policies. To address these gaps, an action plan was implemented, focusing on raising awareness among junior doctors about the importance of risk assessment and anticoagulation. This plan included educational posters displayed in acute care areas, screen savers on hospital computers, educational emails sent to all junior doctors detailing NICE guidelines and local policies, and presentations of the audit results at Medical Grand Rounds and Cardiology department multidisciplinary team (MDT) meetings. A follow-up audit was conducted three months after implementing the improvement plan, involving 50 patients presenting with new or paroxysmal AF during the same period. Data were retrospectively collected using a Performa and analyzed by the hospital’s clinical audit team. This subsequent audit showed a significant improvement in the rates of CHA2DS2-VA and HAS-BLED score assessments, with a 100% rate of correct dosage prescription, demonstrating the effectiveness of the implemented interventions.
Inclusion and exclusion criteria
For the initial and follow-up audits, specific inclusion and exclusion criteria were established to ensure the accuracy and relevance of the data collected. The inclusion criteria required that patients be diagnosed with AF, whether new or paroxysmal, and presenting to acute care units within the hospital, especially non-valvular AF, mitral stenosis in rheumatic heart diseases, and patients with metallic prosthetic valves.[3] This approach ensured that the focus was on managing AF in acute settings. Additionally, patients needed to have accessible and complete medical health records for a thorough retrospective analysis of CHA2DS2-VA and HAS-BLED scores, as well as anticoagulant prescriptions.
Exclusion criteria were set to maintain data integrity and relevance. Individuals whose records lacked complete or reliable data for the required scores or prescriptions were also excluded to avoid inaccuracies. Moreover, patients who did not present to acute care units during the audit period were excluded, as the audit aimed to capture acute care scenarios. Depending on the audit’s focus, patients managed exclusively in outpatient settings might have been excluded to align with the acute care context of the study. These criteria helped ensure that the audit data accurately reflected practices in acute care settings and supported effective improvements in patient management.
Results
The results of the audit include a detailed account of patient demographics, risk scores, anticoagulant prescriptions, and related outcomes. In the dataset, patients varied widely in age, ranging from 22 to 90 years, with a mix of genders. For the CHA2DS2-VASc scores, which assess stroke risk in AF patients, values ranged from 0 to 4, reflecting diverse stroke risk profiles. The HAS-BLED scores, which evaluate bleeding risk, ranged from 0 to 4, indicating varying levels of bleeding risk among patients. For anticoagulation therapy, a variety of medications were prescribed, including Rivaroxaban, Apixaban, and Warfarin, with dosages and durations tailored to individual patient needs. Rivaroxaban was prescribed at 15 mg once daily (HS) for most patients, while apixaban was administered at 2.5 mg or 5 mg twice daily (BD), and Warfarin dosages ranged from 2.5 mg to 7.5 mg daily. The duration of anticoagulation therapy was predominantly indefinite, with a few cases of 6-month courses. International Normalized Ratio (INR) monitoring was required for patients on Warfarin, with regular monitoring indicating adherence to safe dosing practices. Adverse events reported included mild gum bleeding, which occurred in a few cases, but no major bleeding events or other serious complications were noted in most patients. The dataset underscores the variation in anticoagulation management and highlights the implementation of guidelines for assessing stroke and bleeding risks. The audit reflects a commitment to improving patient safety and aligning anticoagulant use with recommended practices, addressing gaps identified in the initial audit, and reinforcing the importance of appropriate risk assessment and monitoring in managing AF patients. In the audit, it was found that CHA2DS2-VA and HAS-BLED scores were calculated for only 27% and 5% of patients, respectively. Anticoagulants were prescribed to 42% of patients, but only 20% of these received the correct dosage. Additionally, 38% of patients did not receive any anticoagulants. In contrast, the re-audit demonstrated a significant improvement in all these metrics. CHA2DS2-VA and HAS-BLED scores were calculated for 100% of patients, and anticoagulant prescriptions reached full compliance at 100%. Moreover, the re-audit showed that 100% of patients received the correct dosage of anticoagulants, and the proportion of patients who did not receive any anticoagulants dropped to 0%.
| Drug | Dosage | Total patients (Audit) |
Adverse events (Audit) |
Total patients (Re-audit) |
Adverse events (Re-audit) |
| Rivaroxaban | 15 mg HS | 10 | 5 | 8 | 4 |
| Apixaban | 2.5 mg BD | 13 | 3 | 17 | 4 |
| Warfarin | 5 mg OD | 10 | 2 | 5 | 2 |
| None | N/A | 17 | 0 | 20 | 0 |
Table 1: Adverse events with the use of anticoagulants in QIP
Examining the specific drug usage and adverse events, rivaroxaban was prescribed to 10 patients in the audit, with 5 reporting adverse events, compared to 8 patients and 4 adverse events in the re-audit. Apixaban was used by 13 patients in the audit, with 3 adverse events, whereas 17 patients received apixaban in the re-audit, with 4 adverse events reported. Warfarin usage decreased from 10 patients in the audit to 5 in the re-audit, with the number of adverse events remaining stable at 2. The number of patients who did not receive any anticoagulants decreased from 17 in the audit to 20 in the re-audit, with no adverse events reported in either period. These results highlight a marked improvement in adherence to guidelines and a decrease in the incidence of adverse events, reflecting the positive impact of the interventions implemented between the audit and re-audit.
| Measure | Audit Results | Re-audit Results |
| CHA2DS2-VA Score Calculated | 27% | 100% |
| HAS-BLED Score Calculated | 5% | 100% |
| Anticoagulants Prescribed | 42% | 100% |
| Correct Dosage | 20% | 100% |
| No Anticoagulants Prescribed |
38% |
0% |
Table 2: Results of Audit and Re-audit
To achieve the remarkable 100% compliance in the re-audit results, a series of targeted strategies and interventions were implemented. Initially, the audit identified significant gaps in the use of CHA2DS2-VASc and HAS-BLED scores, with only 40% and 30% compliance rates, respectively, and a concerning rate of incorrect or absent anticoagulant prescriptions. To address these issues, an extensive awareness campaign was launched, educating junior doctors and healthcare staff about the critical importance of accurate risk assessment and adherence to international guidelines. This campaign included the display of eye-catching posters in acute care areas and the dissemination of educational emails emphasizing the relevance of CHA2DS2-VASc and HAS-BLED scores.
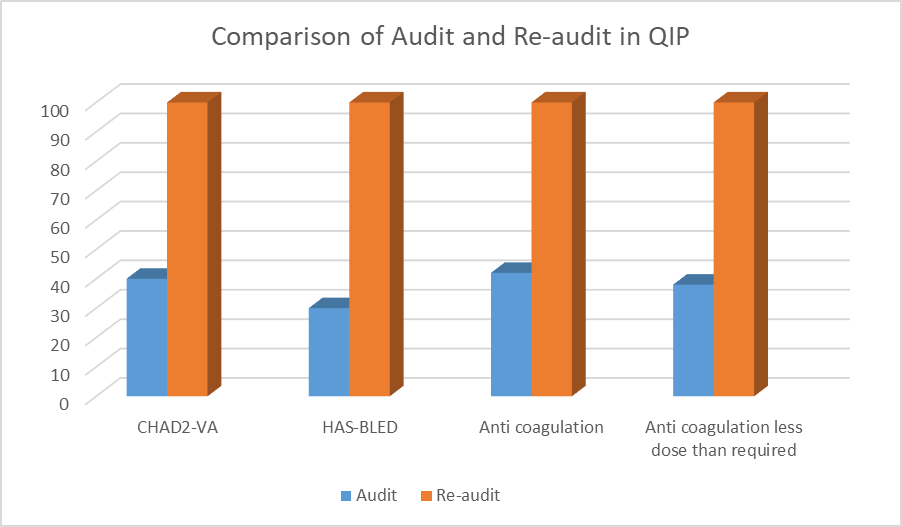
Figure 1: Comparision of audit and re-audit in QIP
These concerted efforts collectively contributed to achieving 100% compliance in the re-audit, highlighting the effectiveness of a multifaceted approach in improving clinical practices. Maintaining this high standard will require ongoing education, adherence to guidelines, and continuous monitoring to ensure sustained excellence in anticoagulant management for AF patients.
In conclusion, the substantial improvements observed from the initial audit to the re-audit reflect a remarkable enhancement in anticoagulant management for AF patients. The increase in compliance with CHA2DS2-VA and HAS-BLED scoring from 40% and 30% to 100%, respectively, and the rise in correct dosage prescriptions from 42% to 100% signify a significant advancement in clinical practice. This progress, alongside the reduction in adverse events, highlights the effectiveness of the implemented changes.
Discussion
This project aimed to enhance the management of anticoagulation in patients with AF within the acute care setting by promoting the routine use of CHA2DS2-VA and HAS-BLED risk scores to guide anticoagulant prescriptions. By focusing on these risk assessment tools, the project successfully increased its application, aligning practices more closely with established guidelines. Specifically, ESC and NICE recommend the routine calculation of CHA2DS2-VA and HAS-BLED scores for all AF patients to inform the decision-making process for anticoagulation therapy.[4] These guidelines are crucial for balancing stroke prevention against the risk of bleeding. However, the project also highlighted several persistent challenges in anticoagulation management in acute settings. Oral anticoagulants are frequently involved in serious medication errors and legal claims, primarily due to the complexities of bleeding risk assessment and the high-pressure environment of acute care. Inadequate skills and a lack of proper tools for risk assessment can result in unsafe prescription practices. Moreover, patient-related factors, such as memory issues, can further complicate the accurate assessment of risk, potentially leading to severe consequences if adequate time for counseling is not provided. The acute care environment, characterized by its fast pace and frequent disruptions, exacerbates these issues, leading to documentation and communication errors that can impact the appropriateness of anticoagulant prescriptions. Despite the project’s success in increasing the use of risk scores, these factors have contributed to a reduction in the rate of correct anticoagulation prescriptions. This underscores the need for ongoing education, improved systems for risk assessment, and better support mechanisms to address the complexities of anticoagulant management, in alignment with NICE and ESC recommendations, and to ensure safer patient outcomes. In addition to raising awareness, dedicated training sessions were organized to enhance staff understanding of these scoring systems, ensuring they were well-versed in their application and impact on patient care. The integration of NICE guidelines and ESC protocols was also prioritized, with updated clinical pathways and decision-support tools embedded in electronic health records (EHRs) to aid clinicians in performing risk assessments and prescribing appropriate therapies.[1] Enhanced documentation practices were established to combat under-documentation, streamlining the workflow for recording risk scores and ensuring their consistent use and accessibility. Regular monitoring and feedback mechanisms were put in place to track compliance rates and address deviations promptly, while real-time feedback from follow-up audits reinforced the importance of adherence to guidelines. Efforts were also made to actively engage patients in their care plans, educating them about the significance of anticoagulation therapy and personalized treatment.
Conclusion
The audit and subsequent re-audit demonstrated a substantial improvement in compliance with CHA2DS2-VASc and HAS-BLED scoring, achieving a commendable 100% adherence rate in the re-audit phase. Initially, the audit highlighted significant gaps in risk assessment and anticoagulant prescription practices, less number of patients receiving the appropriate risk scores, and a troubling proportion of patients not receiving any anticoagulants or receiving incorrect dosages. This performance underscored the critical need for improved adherence to established guidelines. The successful implementation of an awareness campaign, coupled with targeted training and integration of NICE and ESC guidelines into clinical workflows, was instrumental in achieving these results. By focusing on enhancing knowledge, improving documentation practices, and embedding decision-support tools within electronic health records, the project effectively addressed the identified gaps and ensured accurate risk assessment and appropriate anticoagulant therapy. To build on this success and sustain high compliance rates, it is crucial to continue reinforcing the importance of CHA2DS2-VA and HAS-BLED scores in clinical practice. Ongoing education and training for healthcare professionals should be prioritized, with regular updates on guidelines and protocols. Additionally, maintaining robust monitoring systems to track compliance and promptly address any deviations will help ensure continued adherence to best practices. Engaging patients in their care and emphasizing the importance of anticoagulant therapy can further enhance treatment outcomes. By fostering a culture of adherence to guidelines and continuous improvement, the quality of care for AF patients can be significantly elevated, ultimately leading to better patient outcomes and reduced risk of adverse events.
References
- Atrial fibrillation: diagnosis and management. NICE guideline (NG196). Atrial fibrillation: diagnosis and management
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132. doi.10.1016/j.jacc.2019.01.011 PubMed | Crossref | Google Scholar
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373-498. doi.10.1093/eurheartj/ehaa612 PubMed |Croosref | Google Scholar
- Johnson KW, Torres Soto J, Glicksberg BS, et al. Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71(23):2668-2679. doi.10.1016/j.jacc.2018.03.521 PubMed | Crossref | Google Scholar
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. doi.10.1056/NEJMoa0905561 PubMed | Crossref | Google Scholar
Acknowledgments
Not applicable
Funding
Not applicable
Author Information
Corresponding Author:
Mehak Ahsan
Department of Medicine
Bahria International Hospital Rawalpindi, Pakistan
Email: mehakahsan96@yahoo.com
Co-Author:
Hina Ali Akbar
Department of Cardiology
Baba Bulleh Shah DHQ Hospital Kasur, Pakistan
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing, original draft preparation, and writing, review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
Approved by the ethical committee of Baba Bulleh Shah DHQ Hospital, Kasur.
Conflict of Interest Statement
Not reported
Guarantor
Not reported
DOI
Cite this Article
Mehak A, Hina AA. A Quality Improvement Project Focused on Clinical Audit and Re-Audit on the Use of Anticoagulation in Atrial Fibrillation Patients with International Guidelines. medtigo J Med. 2024;2(4):e30622422. doi:10.63096/medtigo30622422 Crossref









